ADVERTISEMENTS:
In this article we will discuss about the water sample collection for physical and chemical analysis.
I. Collection of Water Sample:
A representative sampling is usually done by collecting samples from a number of places from different depths and mixing them together to get a composite sample.
A Kemmerer, Friedinger or Forest type sampler is used for water collection. Bubbling or mixing with air and other gases and undue agitation should be avoided during collection of samples for estimation of dissolved gases.
ADVERTISEMENTS:
The sampler is connected with a flexible tube fixed at its bottom. After collection, the water is let out through the tube which is connected to a glass tube. Water is allowed to fill the sample bottle slowly. In case the sampler is not available, water may be collected in a large beaker or a plastic bucket and transferred to sample bottles by siphon tube.
Precaution:
1. For estimation of dissolved oxygen the water is allowed to overflow at least three times the volume of the sample bottle avoiding air bubbles. The sample for dissolved oxygen (DO) should be fixed immediately after collection and preferably analysed on the spot.
2. Analysis of CO2 should be done immediately after collection.
ADVERTISEMENTS:
3. Free ammonia should be analysed on the spot or fixed with a few drops of sulphuric acid and taken to the laboratory.
4. Total alkalinity should be estimated soon after collection, because loss of CO2 from the water may give different result, if analysed later.
5. Determination of temperature, turbidity and pH should be done on the spot.
6. The materials to be studied later are fixed with few drops of chloroform or toluene and taken to laboratory, but in no case the analysis be delayed for more than 70 hrs.
7. The sample should be kept in dark and at low temperature.
II. Salinity Estimation:
Salinity (S) is the grams of dissolved salts (NaCl) in 1000 g sea-water/water. It is expressed in parts per thousand (%o or ppt), and ranges from 33 to 38%o in the open ocean.
With Salinometer:
Sea-water is an electrolyte and can conduct electric current. Sea-water conductivity is used as an index of salinity. At a given temperature electrical conductivity increases with salinity. Conductivity techniques of salinity estimation are far more accurate then other techniques.
With Refractometer:
ADVERTISEMENTS:
The refractive index of water is directly related to salinity. Modern refractometers are temperature compensated and provide a convenient tool for measuring salinity. The meter gives accurate measurement of salinity percentage in the field.
III. Measurement of Temperature:
The temperature of water bodies is determined with the help of a Celsius thermometer.
Celsius thermometer:
A Celsius thermometer is graduated in 0.1°C td 0.01°C scales.
ADVERTISEMENTS:
Deep the thermometer directly in the sample and read the mercury level. Record the reading of the thermometer in degree Celsius.
Thermistor:
(Fig. 43.1)
Thermistor is a dry cell operated or main line operated digital displaying field instrument. Thermistor is available with a temperature probe, either. Insert the temperature probe in the temperature input socket. Select main or battery as power source. Dip the temperature sensor — a glass globule — into the sample in different depths of water. The temperature is displayed in the digital display meter (potentiometer).
ADVERTISEMENTS:
Read and record the temperature.
Gases:
Solubility of a gas in water depends on the concentration (partial pressure), availability of the gas and temperature. Oxygen is sparingly soluble in water, carbon dioxide is much more soluble, about 30 times more than O2.
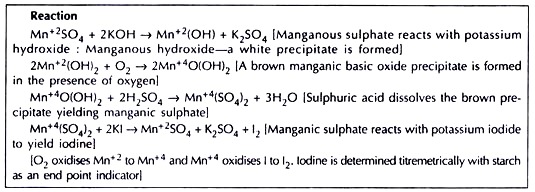
IV. Estimation of Dissolved Oxygen (DO) (Winkler’s Method):
ADVERTISEMENTS:
Reagents:
Manganese sulphate:
400 g MnSO4.2H2O is dissolved in distilled water. Distilled water added to make the volume 1 litre.
Concentrated Sulphuric acid (H2SO4):
Starch indicator:
1 g soluble starch is added to boiling distilled water. On cooling required amount of distilled water is added to make the volume 100 ml. Use freshly prepared starch solution in titration.
ADVERTISEMENTS:
Sodium thiosulphate (0.025 N):
6.205 g thiosulphate (Na2S2O3) is boiled in distilled water, cooled and distilled water added to make the volume 1 litre. The solution is stored in a coloured bottle. 2 to 3 pellets of sodium hydroxide may be added as a preservative.
Alkaline iodide:
150 g potassium iodide (Kl) is dissolved in distilled water. Distilled water added to make the volume 1 litre.
Qualitative Estimation:
ADVERTISEMENTS:
Procedure:
1. Water from the bottom of a sampler is taken in a ground glass stoppered B.O.D. bottle of about 200 ml capacity.
2. Open the sample bottle for a short time and immediately add 1 ml manganous sulphate and 1 ml alkaline iodide in quick succession well below the liquid surface. Re-stopper the bottle, avoid entrapping of air bubbles.
3. Shake the bottle mildly 2-3 times to mix the reagents thoroughly.
4. A precipitate is formed and settles at the bottom indicating presence of D.O.
a. White ppt.: O2 content little.
b. Little brown ppt.: O2 content low.
c. Reddish brown ppt.: O2 content high.
Quantitative Estimation:
Procedure:
Follow the procedure adopted for qualitative estimation (points 1 to 4) resulting in a precipitate of brown manganic basic oxide.
Determine the amount of D.O. in the sample water by titration method:
1. Remove the stopper and add 1 ml concentrated H2SO4.
2. With little shaking of the bottle the ppt. dissolves and a clear straw-yellow solution results.
3. Transfer 100 ml of solution to a 250 ml conical flask for titration.
4. Titrate with 0.025 N sodium thiosulphate solution with the help of a burette. A pale straw- yellow colour appears.
5. Add 3 to 4 drops of one per cent starch solution. The colour turns blue. Continue titration. The blue colour disappears (end point).
Note the reading of the burrete to ascertain the volume of sodium thiosulphate used.
For accurate result repeat titration for several times. Record the readings in data sheet and calculate the mean value.
Data Sheet:
where V1 = Volume of titrant (ml)
N = Normality of titrant (0.025)
V2 = Volume of sampling bottle after placing the stopper (ml)
V3 = Volume of manganous sulphate + potassium iodide solution added (ml)
V4 = Volume of fraction of the contents used for titration (ml)
8 = Equivalent lot of oxygen
0.698 = A factor to convert mg into ml (standardisation value)
V. Estimation of Carbon dioxide (CO2):
Reagents:
NaOH solution (1N):
40 g NaOH (sodium hydroxide) is dissolved in distilled water. Distilled water added to make the volume 1 litre.
Standard sodium Hydroxide … 0.0227N
Phenolphthalein indicator solution
Reaction:
2NaOH + CO2 → Na2CO3 + H2O
Qualitative Estimation:
Procedure:
1. 100 ml water from the bottom of a sampler is transferred to a graduated cylinder through a flexible tube.
2. Add 4 drops of phenolphthalein indicator.
3. pH would be above 8 if the sample turns pink.
Conclusion:
Free carbon dioxide is absent in the sample.
Quantitative Estimation (Titrimetric Method):
Procedure:
Follow the procedure adapted (points 1, 2) for qualitative estimation. Free CO2 is present if the sample remains colourless.
1. Titrate quickly with 0.0227N NaOH solution with the help of a burette, stirring the sample gently with a glass rod.
2. A faint pink colour appears.
Note the reading of the burette to ascertain the volume of sodium hydroxide used.
For accurate result repeat titration for several times. Record readings in data sheet and calculate the mean value.
Data Sheet:
N = Normality of titrant (0.0227)
22 = Equivalent lot of CO2