ADVERTISEMENTS:
Here is a compilation of term papers on ‘Enzymes’ for class 8, 9, 10, 11 and 12. Find paragraphs, long and short term papers on ‘Enzymes’ especially written for school and college students.
Term Paper on Enzymes
Term Paper Contents:
ADVERTISEMENTS:
- Term Paper on the Meaning of Enzymes
- Term Paper on the Characteristics of Enzymes
- Term Paper on the Structure and Functions of Enzymes
- Term Paper on the Classification of Enzymes
- Term Paper on the Properties of Enzymes
- Term Paper on the Factors affecting Enzyme Function
- Term Paper on the Cofactors for Enzymes
Term Paper # 1. Meaning of Enzymes:
An enzyme is a protein that functions as a catalyst to speed up a chemical reaction in the body. It is not used in the chemical reaction rather it is recycled and used over and over again. All enzymes are proteins. Enzymes are biological catalysts. A catalyst speed up reactions. These reactions would take place anyway, the enzymes just speed them up.
Enzymes are proteins that speed up biochemical reactions without being consumed or changed by the reaction. They are found throughout nature; in our bodies, in the environment, and in all living things.
ADVERTISEMENTS:
Without enzymes, life would not be possible. Enzymes speed up chemical and biochemical reactions, and this process is called catalysis. The substances upon which enzymes work when performing catalysis are known as substrates, and each enzyme will only fit and act upon a specific set of substrates.
There are so many enzymes that it would be impossible to name them all. In fact, scientists have yet to discover many enzymes, or fully understand their structure and properties. On the other hand, many other enzymes have been successfully studied and applied to industrial and commercial uses.
Term Paper # 2. Characteristics of Enzymes:
i. Enzymes do not make anything happen that couldn’t happen on its own, just makes it happen faster.
ii. Enzymes are not used in reactions. They can be used over and over again as they remain unchanged in the reaction.
iii. Enzymes are needed in small amounts.
iv. Each enzyme is highly selective about its substrate (substrate specific).
v. Enzymes chemically recognize, bind and modify substrates.
Term Paper # 3. Structure and Function of Enzymes:
ADVERTISEMENTS:
Enzymes are highly specific for a substrate and thus catalyze only one chemical reaction. This substrate specificity is due to 3- dimensional (3-D) structure of the enzyme.
Substrate:
It is the reactant in the chemical reaction that is catalyzed by the enzyme.
Active Site:
ADVERTISEMENTS:
It is the part of the enzymes that bind to the substrate. The shape of the active site determines which substrate binds with that particular enzyme.
ES-Complex:
When the enzyme binds temporarily to the substrate, enzyme -substrate complex is formed.
ADVERTISEMENTS:
Activation energy is required for the chemical process to occur. At the end of the chemical process, the activation energy is reduced. New product or products are formed after the completion of the reaction and the enzyme is released to be reused.
Lock and Key Model:
Enzyme specificity is often described using lock and key model. The shape of the active site (lock) determines which substrate (key) will fit into the enzyme. If the substrate cannot fit into the active site, the enzyme cannot catalyze the chemical reaction.
Term Paper # 4. Classification of Enzymes:
ADVERTISEMENTS:
Thousands of enzymes have been discovered, isolated and studied. Most of these enzymes have been classified into different groups based on the type of reactions they catalyze.
Enzymes are divided into 6 classes each with 4-13 subclasses and named accordingly by a four-digit number:
1. Oxidoreductases/Dehydrogenases:
Enzymes which catalyze oxidoreduction between two substrates For example, S and S’
2. S reduced + S’ oxidised → – S oxidised + S’ reduced
ADVERTISEMENTS:
Transferases:
Enzymes that catalyze a transfer of a group.
For example, G (other than hydrogen) between a pair of substrate S and S’
S – G + S’ → – S + S’ – G
3. Hydrolases:
Enzymes that catalyze hydrolysis of ester.
ADVERTISEMENTS:
For example, ether, peptide, glycosidic, C-C, C-halide or P-N bonds.
4. Lyases:
Enzymes that catalyze removal of groups from substrates by mechanisms other than hydrolysis leaving double bonds.
For example:
5. Isomerases:
All enzymes that catalyze inter-conversion of optical, geometric or positional isomers.
6. Ligases:
Enzymes that catalyze the linking together of 2 compounds
For example, enzymes which catalyze joining of C-O, C-S, C-N, P-O etc. bonds.
Term Paper # 5. Properties of Enzymes:
The enzymes possess many outstanding properties, which can be enumerated as given below:
(i) Enzymes are Colloidal in Nature:
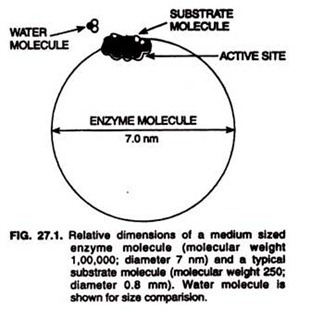
Enzyme molecules are of giant size. Their molecular weight range from 12,000 to 1 million. They are, therefore, very large compared with the substrates or functional group they act upon (Fig. 27.1) It has been observed that the molecular weight of many enzymes prove to be approximately an n- fold multiple (where n is an integer) of 17,500 which is found to be an unit in most proteins.
On account of their large size, however, the enzyme molecules show extremely low rates of their diffusion and form colloidal systems in water. Being colloidal in nature, the enzymes are non-dialyzable although some contain dialyzable or dissociable component in the form of coenzymes.
(ii) Enzymes are Catalytic in Nature:
The enzymes catalyze and therefore accelerate the rate of chemical reactions. They do not normally participate in these reactions or if they do so, at the end of reaction, they are recovered as such without undergoing any qualitative or quantitative change. It is this property of enzymes that helps them catalyze the transformation of a large quantity of substrate.
The catalytic power of an enzyme is measured by the “turnover number” that refers to the number of substrate molecules converted into product time, when the enzyme is fully saturated with substrate. The maximum turnover numbers of some enzymes is given in Table 27.2.
(iii) Most Enzymes are Proteins:
With the exception of a small group of catalytic RNA molecules called ribozymes, all enzymes are proteins. Their catalytic activity depends on the integrity of their native protein conformation because all the primary, secondary, tertiary, and quaternary structures of protein enzymes are essential to other catalytic activity. If an enzyme is broken down into its component amino acids, its catalytic activity is always destroyed.
(iv) Enzymes are Highly Specific in their Action:
Enzymes are highly specific in their action, with few exceptions.
Their specificity lies in the fact that they may act:
a. On the specific type of substrate molecule or
b. On a group of structurally related compounds or
c. On only one of the two optical isomers of a compound or
d. On only one of the two geometrical isomers. Accordingly, four patterns of enzyme specificity have been recognised.
a. Absolute specificity:
Some enzymes act on only one substrate. For instance, carbonic anhydrase acts to bring about the union of CO2 with water to form carbonic acid.
b. Group specificity:
Certain enzymes catalyze the reaction of a structurally-related group of compounds. For example; lactic dehydrogenase (LDH) catalyzes the inter-conversion of pyruvic and lactic acids and also a number of other structurally related compounds.
c.Optical specificity:
Optical specificity is the most striking aspect of enzyme specificity. In this case a particular enzyme acts with only one of the two optical isomers of a compound. For example, arginase reacts with only L-arginine and not with its D-isomer. However, some enzymes are specific to interconvert the two optical isomers of a compound. For instance, alanine recemase catalyzes the inter-conversion between L- and D-alanine.
d. Geometrical specificity:
Certain enzymes show specificity only towards the cis and trans forms of a compound. For example, the enzyme fumarase catalyses the interconversion of fumaric acid and L-malic acid. It does not act on the maleic acid, the cis isomer of fumaric acid, or with D-malic acid.
(v) Enzymes are of Amphoteric Nature:
Each enzyme molecule possesses numerous groups, which yield hydrogen ions (H ions) in slightly alkaline solutions, and groups that yield hydroxyl ions (OH–ions) in slightly acidic solutions. That is, the enzymes are capable of ionising either as an acid or as a base depending upon the acidity of the external solution.
(vi) Enzymes Show Reversibility:
Enzymes have ability to accelerate the chemical reactions in either direction, i.e., onward and backward direction, depending upon the availability of suitable energy sources.
For example:
(vii) Enzymes Alter Only the Reaction Rate and not the Reaction Equilibrium:
Enzyme catalysts enhance reaction rates by lowering activation energies; they are no exception to the rule that catalysts do not affect reaction equilibria. For instance, consider the inter-conversion of S(substrate) and P (product). Suppose that, in the absence of enzyme, the forward rate constant (kF) of the reaction is 10-4s-1 and the reverse rate constant (kR) is 10-6s-1.
The equilibrium constant K is given by the ratio of these constants:
The equilibrium concentration of P is 100 times that of S, whether or not enzyme is present. However, it might take considerable time to approach this equilibrium without enzyme, whereas equilibrium would be attained rapidly in the presence of a suitable enzyme due to increased reaction rate. That is, the enzymes accelerate the attainment of equilibrium but do not shift their positions. The equilibrium position is a function only of the free energy difference between reactants and products.
(viii) Enzymes can be regulated:
If excess of the end-product is made, which is against the cell economy, the activity of the enzyme is slowed down by the interaction of the end-product with the enzyme. This phenomenon is called feedback inhibition.
(ix) Enzymes Enhance Reaction Rates at Ordinary Room Temperature and Normal Atmospheric Pressure:
In contrast to inorganic catalysts, which usually require very high temperatures (temp, exceeding 500°C) and several atmosphere of pressure, the enzymes catalyse the same reaction at ordinary room temperature and normal atmospheric pressure.
Term Paper # 6. Factors affecting Enzyme Function:
Factors that affects enzyme functions are given below:
(i) Temperature:
Enzyme functions optimally at certain temperature. As the temperature increases the kinetic energy also increases, molecules move faster and it increases the chance of substrate colliding the enzyme’s active site and binding followed by reaction.
But if the temperature is too high, the enzyme protein denatures (cooks) thereby destroying the shape of active binding site (cannot bind to the substrate anymore) and decreasing the enzyme reaction rate.
(ii) pH (Measure of Acidity):
Enzymes function optimally at certain pH and are certainly sensitive to the changes in the pH. Changes in the pH can make or break chemical bonds in the active binding site and thereby decreasing its effectiveness. If the pH is too high (acidic) or low (basic), the enzyme denatures. However there are exceptions, digestive enzymes in the stomach function at pH of 3-4.
(iii) Concentration of the Substrate:
As the enzyme concentration increases, the rate of reaction also increases until a point when the amount of substrate available becomes limited. Similarly, when the substrate concentration is low, the rate of reaction is also slow.
Term Paper # 7. Cofactors for Enzymes:
Simple enzymes, which are wholly made up of protein, are catalytically active only with their amino acid residues, while the conjugate enzymes require an additional non-protein chemical component for catalytic activity. This additional non-protein chemical component is called a cofactor. Cofactors can be subdivided into two groups: inorganic elements (metal ions) and small organic molecules (coenzymes).
Inorganic elements (metal ions) or small organic molecules (coenzymes) may be loosely or tightly (even covalently) bound to the enzyme. A tightly or even covalently bound metal ion or coenzyme to enzyme is called a prosthetic group. For instance, the nucleotides (NAD+ and NADP+) and flavin nucleotides (FMN and FAD) are coenzymes.
NAD+ and NADP+ are loosely bound to an enzyme and move readily from one enzyme to another; FMN and FAD are usually very tightly bound to the enzymes, called flavoproteins, for which they serve as prosthetic groups. A complete, catalytically active enzyme together with its bound cofactor (either metal ion and /or coenzyme) is referred to as a holoenzyme. The protein part of such enzyme is called the apoenzyme or apoprotein.
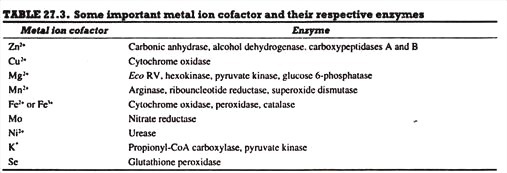
Inorganic Elements or Metal Ions:
Various inorganic elements or metal ions such as Fe2+, Fe3+, Zn2+, Mg2+, Mo, etc. serve as cofactors for enzymes. Each enzyme may require either one or more inorganic elements as cofactor for its catalytic activity. In fact, more than one third of all enzymes known either contain bound metal ions or require the addition of such ions for their catalytic activity.
The chemical reactivity of metal ions-associated with their positive charges, with their ability to form relatively strong yet kinetically labile bonds, and, in some cases, with their capacity to be stable in more than one oxidation state—explains why catalytic strategies that employ metal ions have been adopted throughout evolution. However, some important metal ion cofactors and the respective enzymes are listed in Table 27.3.
Coenzymes:
Cofactor that are small organic molecules are called coenzymes. Coenzymes, like metal ions, may be either tightly bound (prosthetic group) or loosely bound to the enzyme. The loosely bound coenzymes are more like ‘cosubstrates’ as they bind to and are released from the enzyme just as substrates and products are.
Co-enzymes are organic compounds and their association with the apo enzyme is only during the course of catalysis. Furthermore, co-enzymes serve as co-factors in a number of different enzyme catalyzed reactions.
The essential chemical components of many co-enzymes are vitamins, e.g., coenzyme nicotinamide adenine dinucleotide (NAD) and NADP contain the vitamin niacin. A number of enzymes require metal ions for their activity. This activity forms coordination bonds with side chains at the active site and at the same time, forms one or more coordination bonds with the substrate.
For example, zinc is a cofactor for the proteolytic enzyme – carboxypeptidase. Catalytic activity is lost when the co-factor is removed from the enzyme which testifies that co-factors play a crucial role in the catalytic activity of the enzyme.
But, in fact, they are not co-substrates because of the use of the same coenzyme by a variety of enzymes and their source in vitamins (coenzymes are often derived from vitamins). However, some important coenzymes and the respective enzymes are listed in Table 27.4.
1. Coenzymes are often derived from vitamins:
Many of the coenzymes, the small organic molecules, are derived from vitamins. Vitamins themselves are organic molecules and play significant roles in nearly all forms of life. Plants, fungi, and bacteria synthesize the vitamins they need, but higher animals (including humans) have lost this capacity in the course of evolution and require vitamins in their diets.
For example, Escherichia coli can thrive on glucose and organic salts and can synthesize its all required vitamins, humans require at least 12 vitamins in the diet.
Vitamins can be grouped according to whether they are soluble in water or in nonpolar solvents. Water soluble vitamins usually function as coenzymes. All members of vitamin B series, the series called vitamin B complex, are water soluble and serve as the components of coenzymes (Table 27.5).
2. Coenzymes function as transient carriers:
Coenzymes function as transient carriers of specific atoms or functional groups. Nicotinamide adenine dinucleotide (NAD+), nicotinamide adenine dinucleotide phosphate (NADP+), flavin adenine dinucleotide (FAD), and flavin mononucleotide (FMN) are water soluble coenzymes and act as universal electron carriers. Both NAD+ and NADP+ coenzymes, for example, undergo reversible reduction of the nicotinamide ring.
As a substrate molecule undergoes oxidation (dehydrogenation), giving up two hydrogen atoms, the oxidised form of the nucleotide (NAD+ or NADP+) accepts a hydride ion (: H– , the equivalent of a proton and two electrons) and is transformed into the reduced form (NADH or NADPH). The second proton removed from the substrate is released to the aqueous solvent.
The half reaction for each type of nucleotide is therefore:
NAD+ + 2e– + 2H+ →NADH + H+
NADP+ + 2e– + 2H+ → NADPH + H+
Table 27.6:
Lists some coenzymes that serve as transient carriers of specific atoms or functional groups.
The catalytic activity of an enzyme depends on the presence of small molecules called as cofactors which activate enzyme. An enzyme without its cofactor is termed as Apo enzyme.
Apo enzyme + Cofactor = Holoenzyme
kinds of cofactors – prosthetic groups, co-enzymes and metal ions.