ADVERTISEMENTS:
Read this article to learn about the aspects, applications and limitations of somatic hybridization.
The somatic hybridization involves three aspects. The three aspects are: (A) Fusion of Protoplasts (B) Selection of Hybrid Cells and (C) Identification of Hybrid Plants.
The conventional method to improve the characteristics of cultivated plants, for years, has been sexual hybridization. The major limitation of sexual hybridization is that it can be performed within a plant species or very closely related species. This restricts the improvements that can be done in plants.
ADVERTISEMENTS:
The species barriers for plant improvement encountered in sexual hybridization can be overcome by somatic cell fusion that can form a viable hybrids. Somatic hybridization broadly involves in vitro fusion of isolated protoplasts to form a hybrid cell and its subsequent development to form a hybrid plant.
Plant protoplasts are of immense utility in somatic plant cell genetic manipulations and improvement of crops. Thus, protoplasts provide a novel opportunity to create cells with new genetic constitution. And protoplast fusion is a wonderful approach to overcome sexual incompatibility between different species of plants. More details on the applications of somatic hybridization are given later.
Somatic hubridization involves the following aspects:
A. Fusion of protoplasts
ADVERTISEMENTS:
B. Selection of hybrid cells
C. Identification of hybrid plants.
A. Fusion of Protoplasts:
As the isolated protoplasts are devoid of cell walls, there in vitro fusion becomes relatively easy. There are no barriers of incompatibility (at interspecific, inter-generic or even at inter-kingdom levels) for the protoplast fusion. Protoplast fusion that involves mixing of protoplasts of two different genomes can be achieved by spontaneous, mechanical, or induced fusion methods.
Spontaneous fusion:
Cell fusion is a natural process as is observed in case of egg fertilization. During the course of enzymatic degradation of cell walls, some of the adjoining protoplasts may fuse to form homokaryocytes (homokaryons). These fused cells may sometimes contain high number of nuclei (2-40).
This is mainly because of expansion and subsequent coalescence of plasmodermal connections between cells. The frequency of homokaryon formation was found to be high in protoplasts isolated from dividing cultured cells. Spontaneously fused protoplasts, however, cannot regenerate into whole plants, except undergoing a few cell divisions.
Mechanical fusion:
The protoplasts can be pushed together mechanically to fuse. Protoplasts of Lilium and Trillium in enzyme solutions can be fused by gentle trapping in a depression slide. Mechanical fusion may damage protoplasts by causing injuries.
ADVERTISEMENTS:
Induced fusion:
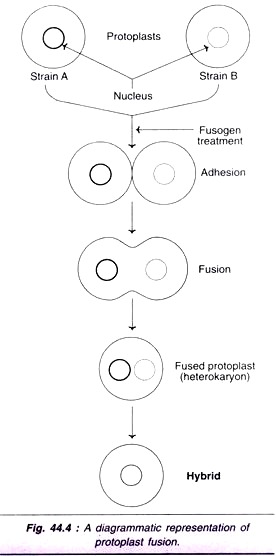
Freshly isolated protoplasts can be fused by induction. There are several fusion-inducing agents which are collectively referred to as fusogens e.g. NaN03, high pH/Ca2+, polyethylene glycol, polyvinyl alcohol, lysozyme, concavalin A, dextran, dextran sulfate, fatty acids and esters, electro fusion. Some of the fusogens and their use in induced fusion are described. A diagrammatic representation of protoplast fusion is depicted in Fig. 44.4.
Treatment with sodium nitrate:
ADVERTISEMENTS:
The isolated protoplasts are exposed to a mixture of 5.5% NaNO3 in 10% sucrose solution. Incubation is carried out for 5 minutes at 35°C, followed by centrifugation (200 x g for 5 min). The protoplast pellet is kept in a water bath at 30°C for about 30 minutes, during which period protoplast fusion occurs. NaNO3 treatment results in a low frequency of heterokaryon formation, particularly when mesophyll protoplasts are fused.
High pH and high Ca2+ ion treatment:
This method was first used for the fusion of tobacco protoplasts, and is now in use for other plants also. The method consists of incubating protoplasts in a solution of 0.4 M mannitol containing 0.05 M CaCI2 at pH 10.5 (glycine-NaOH buffer) and temperature 3 7°C for 30-40 minutes. The protoplasts form aggregates, and fusion usually occurs within 10 minutes. By this method, 20-50% of the protoplasts are involved in fusion.
ADVERTISEMENTS:
Polyethylene glycol (PEG) treatment:
This has become the method of choice, due to its high success rate, for the fusion of protoplasts from many plant species. The isolated protoplasts in culture medium (1 ml) are mixed with equal volume (1 ml) of 28-56% PEG (mol. wt. 1500-6000 Daltons) in a tube. PEG enhances fusion of protoplasts in several species. This tube is shaken and then allowed to settle. The settled protoplasts are washed several times with culture medium.
PEG treatment method is widely used protoplast fusion as it has several advantages:
i. It results in a reproducible high-frequency of heterokaryon formation.
ADVERTISEMENTS:
ii. Low toxicity to cells.
iii. Reduced formation of bi-nucleate heterokaryons.
iv. PEG-induced fusion is non-specific and therefore can be used for a wide range of plants.
Electro-fusion:
In this method, electrical field is used for protoplast fusion. When the protoplasts are placed in a culture vessel fitted with micro- electrodes and an electrical shock is applied, protoplasts are induced to fuse. Electro-fusion technique is simple, quick and efficient and hence preferred by many workers.
Further, the cells formed due to electro-fusion do not show cytotoxic responses as is the case with the use of fusogens (including PEG). The major limitation of this method is the requirement of specialized and costly equipment.
ADVERTISEMENTS:
Mechanism of fusion:
The fusion of protoplasts involves three phases agglutination, plasma membrane fusion and formation of heterokaryons.
1. Agglutination (adhesion):
When two protoplasts are in close contact with each other, adhesion occurs. Agglutination can be induced by fusogens e.g. PEG, high pH and high Ca2+.
2. Plasma membrane fusion:
Protoplast membranes get fused at localized sites at the points of adhesion. This leads to the formation of cytoplasmic bridges between protoplasts. The plasma membrane fusion can be increased by high pH and high Ca2+, high temperature and PEC, as explained below.
ADVERTISEMENTS:
(a) High pH and high Ca2+ ions neutralize the surface charges on the protoplasts. This allows closer contact and membrane fusion between agglutinated protoplasts.
(b) High temperature helps in the intermingling of lipid molecules of agglutinated protoplast membranes so that membrane fusion occurs.
(c) PEG causes rapid agglutination and formation of clumps of protoplasts. This results in the formation of tight adhesions of membranes and consequently their fusion.
3. Formation of heterokaryons:
The fused protoplasts get rounded as a result of cytoplasmic bridges leading to the formation of spherical homokaryon or heterokaryon.
B. Selection of Hybrid Cells:
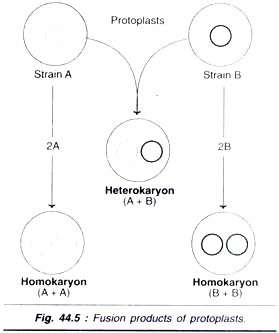
About 20-25% of the protoplasts are actually involved in the fusion. After the fusion process, the protoplast population consists of a heterogenous mixture of un-fused chloroplasts, homokaryons and heterokaryons (Fig. 44.5). It is therefore necessary to select the hybrid cells (heterokaryons). The commonly used methods employed for the selection of hybrid cells are biochemical, visual and cytometric methods.
Biochemical methods:
The biochemical methods for selection of hybrid cells are based on the use of biochemical compounds in the medium (selection medium). These compounds help to sort out the hybrid and parental cells based on their differences in the expression of characters.
Drug sensitivity and auxotrophic mutant selection methods are described below:
1. Drug sensitivity:
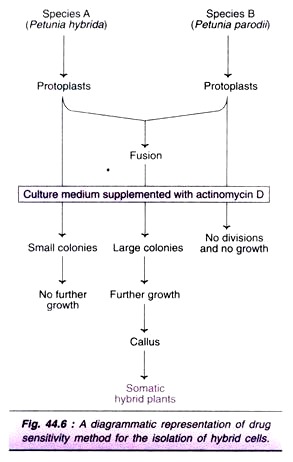
This method is useful for the selection hybrids of two plant species, if one of them is sensitive to a drug. Protoplasts of Petunia hybride (species A) can form macroscopic callus on MS medium, but are sensitive to (inhibited by) actinomycin D. Petunia parodii protoplasts (species B) form small colonies, but are resistant to actinomycin D.
When these two species are fused, the fused protoplasts derive both the characters — formation of macroscopic colonies and resistance to actinomycin D on MS medium. This helps in the selection of hybrids (Fig. 44.6). The parental protoplasts of both the species fail to grow. Protoplasts of P. parodii form very small colonies while that of P. hybrida are inhibited by actinomycin D.
Drug sensitivity technique was originally developed by Power et al (1976) for the selection of hybrids of Petunia sp. A similar procedure is in use for the selection of other somatic hybrids e.g., hybrids between Nicotiana Silvestre’s and Nicotiana knightiana.
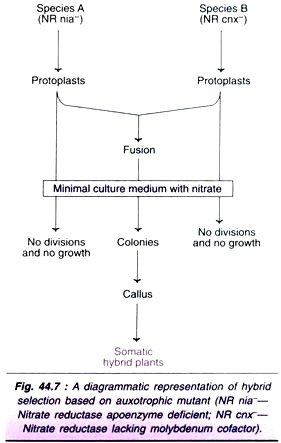
2. Auxotrophic mutants:
Auxotroph’s are mutants that cannot grow on a minimal medium and therefore require specific compounds to be added to the medium. Nitrate reductase deficient mutants of tobacco (N. tabacum) are known. The parental protoplasts of such species cannot grow with nitrate as the sole source of nitrogen while the hybrids can grow.
Two species of nitrate reductase deficiency— one due to lack of apoenzyme (nia-type mutant) and the other due to lack of molybdenum cofactor (cnx- type mutant) are known. The parental protoplasts cannot grow on nitrate medium while the hybrid protoplasts can grow (Fig. 44.7).
The selection of auxotrophic mutants is possible only if the hybrid cells can grow on a minimal medium. Another limitation of the technique is the paucity of higher plant auxotroph’s.
Visual methods:
Visual selection of hybrid cells, although tedious is very efficient. In some of the somatic hybridization experiments, chloroplast deficient (albino or non-green) protoplasts of one parent are fused with green protoplasts of another parent.
This facilitates the visual identification of haterokaryons under light microscope. The heterokaryons are bigger and green in colour while the parental protoplasts are either small or colourless. Further identification of these heterokaryons has to be carried out to develop the specific hybrid plant.
There are two approaches in this direction — growth on selection medium, and mechanical isolation.
1. Visual selection coupled with differential media growth:
There exist certain natural differences in the sensitivity of protoplasts to the nutrients of a given medium. Thus, some media can selectively support the development of hybrids but not the parental protoplasts. A diagrammatic representation of visual selection coupled with the growth of heterokaryons on a selection medium is given in Fig. 44.8.
2. Mechanical isolation:
The visually identified heterokaryons under the microscope can be isolated by mechanical means. This involves the use of a special pipette namely Drummond pipette. The so isolated heterokaryons can be cloned to finally produce somatic hybrid plants. The major limitation of this method is that each type of hybrid cell requires a special culture medium for its growth. This can be overcome by employing micro drop culture of single cells using feeder layers .
Cytometric methods:
Some workers use flow cytometry and fluorescent-activated cell sorting techniques for the analysis of plant protoplasts while their viability is maintained. The same techniques can also be applied for sorting and selection of heterokaryons. The hybrid cells derived from such selections have proved useful for the development of certain somatic hybrid plants.
C. Identification of Hybrid (Cells) Plants:
The development of hybrid cells followed by the generation of hybrid plants requires a clear proof of genetic contribution from both the parental protoplasts. The hybridity must be established only from euploid and not from aneuploid hybrids. Some of the commonly used approaches for the identification of hybrid plants are briefly described.
Morphology of hybrid plants:
Morphological features of hybrid plants which usually are intermediate between two parents can be identified. For this purpose, the vegetative and floral characters are considered. These include leaf shape, leaf area, root morphology, flower shape, its structure, size and colour, and seed capsule morphology.
The somatic hybrids such as pomatoes and topatoes which are the fused products of potato and tomato show abnormal morphology, and thus can be identified. Although the genetic basis of the morphological characters has not been clearly known, intermediate morphological features suggest that the traits are under the control of multiple genes. It is preferable to support hybrid morphological characters with evidence of genetic data.
Isoenzyme analysis of hybrid plants:
The multiple forms of an enzyme catalysing the same reaction are referred to as isoenzymes. Electrophoretic patterns of isoenzymes have been widely used to verify hybridity. Somatic hybrids possess specific isoenzymes (of certain enzymes) of one or the other parent or both the parents simultaneously.
There are many enzymes possessing unique isoenzymes that can be used for the identification of somatic hybrids e.g. amylase, esterase, aspartate aminotransferase, phosphodiesterase, isoperoxidase, and hydrogenases (of alcohol, lactate, malate). If the enzyme is dimeric (having two subunits), somatic hybrids usually contain an isoenzyme with an intermediate mobility properties. The isoenzymes are often variable within the same plant. Therefore, it is necessary to use the same enzyme from each plant (parents and somatic hybrids), from a specific tissue with the same age.
Chromosomal constitution:
The number of chromosomes present in the hybrid cells can be directly counted. This provides information on the ploidy state of the cells. The somatic hybrids are expected to possess chromosomes that are equal to the total number of chromosomes originally present in the parental protoplasts. Sometimes, the hybrids are found to contain more chromosomes than the total of both the parents. The presence of chromosomal markers is greatly useful for the genetic analysis of hybrid cells.
Molecular techniques:
Many recent developments in molecular biology have improved the understanding of genetic constitution of somatic plant hybrids.
Some of them are listed below:
1. Differences in the restriction patterns of chloroplast and mitochondrial DNAs.
2. Molecular markers such as RFLP, AFLP, RAPD and microsatellites.
3. PCR technology.
Chromosome Number in Somatic Hybrids:
The chromosome number in the somatic hybrids is generally more than the total number of both of the parental protoplasts.
However, wide variations are reported which may be due to the following reasons:
1. Fusion of more than two protoplasts.
2. Irregularities in mitotic cell divisions.
3. In fusogen or electro-induced fusions, about one third of the fusions occur between more than two protoplasts.
4. Differences in the status of protoplasts (actively dividing or quiescent) from the two species of plants result in formation of asymmetric hybrids.
5. Asymmetric hybrids may be due to unequal replication of DNA in the fusing protoplasts.
6. Protoplast isolation and culture may also lead to somaclonal variations, and thus variations in chromosome number.
A selected list of interspecific hybrids produced through protoplast fusion along with the number of chromosomes in the hybrids is given in Table 44.3.
Symmetric and asymmetric hybrids:
If the chromosome number in the hybrid is the sum of the chromosomes of the two parental protoplasts, the hybrid is said to be symmetric. Symmetric hybrids between incompatible species are usually sterile. This may be due to production of 3n hybrids by fusing 2n of one species with n of another species.
Asymmetric hybrids have abnormal or wide variations in the chromosome number than the exact total of two species. These hybrids are usually formatted with full somatic complement of one parental species while all or nearly all of the chromosomes of other parental species are lost during mitotic divisions. Asymmetric hybrids may be regarded as cybrids but for the introgressed genes.
As given in Table 44.3, protoplast fusion between N. tabacum (2n = 48) and N. nesophila (2n = 24) results in a symmetric hybrids, while asymmetric hybrids are formed when B. napus and B. junea are fused.
Cybrids:
The cytoplasmic hybrids where the nucleus is derived from only one parent and the cytoplasm is derived from both the parents are referred to as cybrids. The phenomenon of formation of cybrids is regarded as cybridization. Normally, cybrids are produced when protoplasts from two phytogenetically distinct species are fused. Genetically, cybrids are hybrids only for cytoplasmic traits.
Hybrids and Somatic Incompatibility:
Many a times, production of full-pledged hybrids through fusion of protoplasts of distantly related higher plant species is rather difficult due to instability of the two dissimilar genomes in a common cytoplasm. This phenomenon is referred to as somatic incompatibility.
Hybrids formed despite somatic incompatibility may exhibit structural and developmental abnormalities. Several generations may be required to eliminate the undesirable genes. Due to this limitation in somatic hybridization, cybridization involving protoplast fusion for partial genome transfer is gaining importance in recent years.
Methodology of Cybridization:
A diagrammatic representation of the formation of hybrids and cybrids is given in Fig. 44.9.
As the formation of heterokaryon occurs during hybridization, the nuclei can be stimulated to segregate so that one protoplast contributes to the cytoplasm while the other contributes nucleus alone (or both nucleus and cytoplasm). In this way cybridization can be achieved.
Some of the approaches of cybridization are given hereunder:
1. The protoplasts of cytoplasm donor species are irradiated with X-rays or Ƴ-rays. This treatment renders the protoplasts inactive and non-dividing, but they are efficient donors of cytoplasmic constituents when fused with recipient protoplasts.
2. Normal protoplasts can be directly fused with enucleated protoplasts. Enucleated protoplasts can be isolated by high-speed centrifugation.
3. Protoplasts are inactivated by metabolic inhibitors such as iodoacetate. In practice, iodoacetate treated protoplasts are fused with X-rays irradiated protoplasts for more efficient formation of cybrids.
4. It is possible to suppress nuclear division in some protoplasts and fuse them with normal protoplasts.
Genetic recombination in Asexual or Sterile Plants:
There are many plants that cannot reproduce sexually. Somatic hybridization is a novel approach through which two parental genomes of a sexual or sterile plants can be brought together. Thus, by fusing parental protoplasts, fertile diploids and polyploidy can be produced.
Overcoming Barriers of Sexual Incompatibility:
Sexual crossing between two different species (interspecific) and two different genus (inter-generic) is impossible by conventional breeding methods. Somatic hybridization overcomes the sexual incompatibility barriers.
Two examples are given hereunder:
1. Fusion between protoplasts of potato (Solanum tuberosum) and tomato (Lycopersicon esculentum) has created pomato (Solanopersicon, a new genus).
2. Interspecific fusion of four different species of rice (Oryza brachyantha, O. elchngeri, O. officinalis and O. perrieri) could be done to improve the crop.
A list of selected examples of somatic hybrids developed by interspecific protoplast fusion is given in Table 44.4.
A Novel Approach for Gene Transfer:
Somatic hybridization has made it possible to transfer several desirable genetic characters among the plants (Table 44.5).
Applications of Cybrids:
Cybridization is a wonderful technique wherein the desired cytoplasm can be transferred in a single step. Cybrids are important for the transfer of cytoplasmic male sterility (CMS), antibiotic and herbicide resistance in agriculturally useful plants.
Some of the genetic traits in certain plants are cytoplasmically controlled. This includes some types of male sterility, resistance to certain antibiotics and herbicides. A selected list of agronomic characters transferred through cybrids is given in Table 44.5 (along with somatic hybrids).
Cybridization has been successfully used to transfer CMS in rice. Cybrids of Brassica raphanus that contain nucleus of B. napus, chloroplasts of atrazinc resistant B. campestris and male sterility from Raphanus sativas have been developed.
Applications of Somatic Hybridization:
Somatic hybridization has opened new possibilities for the in vitro genetic manipulation of plants to improve the crops.
Some of the practical application are briefly given:
1. Disease resistance:
Several interspecific and inter-generic hybrids with disease resistance have been created. Many disease resistance genes (e.g., tobacco mosaic virus, potato virus X, club rot disease) could be successfully transferred from one species to another. For example, resistance has been introduced in tomato against diseases such as TMV, spotted wilt virus and insect pests.
2. Environmental tolerance:
The genes responsible for the tolerance of cold, frost and salt could be successfully introduced through somatic hybridization, e.g., introduction of cold tolerance gene in tomato.
3. Quality characters:
Somatic hybrids for the production of high nicotine content, and low erucic acid have been developed (Table 44.5).
4. Cytoplasmic male sterility:
ADVERTISEMENTS:
A modification of hybridization in the form of cybridization has made it possible to transfer cytoplasmic male sterility.
Other Application of Somatic Hybridization:
1. Somatic hybridization has helped to study the cytoplasmic genes and their functions. In fact, the information is successfully used in plant breeding programmes.
2. Protoplast fusion will help in the combination of mitochondria and chloroplasts to result in a unique nuclear-cytoplasmic genetic combination.
3. Somatic hybridization can be done in plants that are still in juvenile phase.
4. Protoplast transformation (with traits like nitrogen fixation by incorporating exogenous DNA) followed by somatic hybridization will yield innovative plants.
Limitations of Somatic Hybridization:
Although somatic hybridization is a novel approach in plant biotechnology, there are several problems and limitations.
The success of the technique largely depends on overcoming these limitations, some of which are listed below:
1. Somatic, hybridization does not always produce plants that give fertile and visible seeds.
2. Regenerated plants obtained from somatic hybridization are often variable due to somaclonal variations, chromosomal elimination, organelle segregation etc.
3. Protoplast culture is frequently associated with genetic instability.
4. Protoplast fusion between different species/genus is easy, but the production of viable somatic hybrids is not possible in all instances.
5. Some of the somatic hybrids, particularly when produced by the fusion of taxonomically different partners, are unbalanced and not viable.
6. There are limitations in the selection methods of hybrids, as many of them are not efficient.
7. There is no certainty as regards the expression of any specific character in somatic hybridization.
8. Somatic hybridization between two diploids results in the formation of an amphidiploid which is not favourable. For this reason, haploid protoplasts are recommended in somatic hybridization.