ADVERTISEMENTS:
In this article we will discuss about:- 1. Occurrence and Distribution of Marsilea 2. Sporophyte of Marsilea 3. Gametophyte 4. Phylogeny.
Occurrence and Distribution of Marsilea:
The genus (Marselia) owes its name to Carl Linnaeus (1753) who named it after the Italian count Luigi Ferdinando Marsigli. It is one of the most widely distributed among ferns. But the genus has a preference to the warmer parts of the world. The plants grow in aquatic (M.quadrifolia) or semiterrestrial (M.condensata) habitats. A few species are known to grow in dry soil.
The genus consists of about 53 species of which about nine are recorded from India. Launert (1984) has reported 26 species of Marselia from Africa. Some of the common species are M.quadrifolia, M.hirsuta, M.minuta, M.vestita, M.aegyptiaca etc.
Sporophyte of Marsilea:
ADVERTISEMENTS:
Morphology of the Plant:
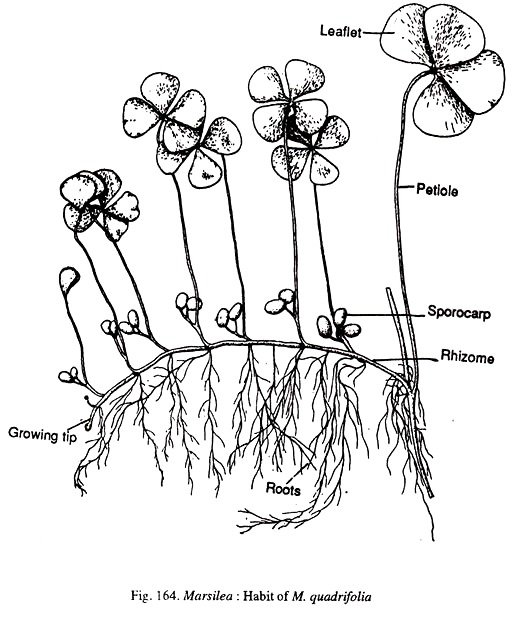
The plant body consists of a creeping cylindrical rhizome which is partly or completely subterranean (Fig.164). The rhizome has an indefinite length which varies with the growth conditions and under luxuriant growth conditions it may reach a length of 25 metres. Branching in the rhizome is quite frequent and it may be axillary or lateral.
The rhizome is distinguished into nodes and internodes. The nodal regions are slightly swollen. The internodes are longer in aquatic species (M.quadrifolia) and shorter in terrestrial species. The leaves and roots are generally borne at the nodes.
ADVERTISEMENTS:
The leaves have a long and slender petiole. In aquatic species only the lamina floats on the surface of water. The leaves are arranged alternately in two rows on the upper surface of the rhizome. The leaves are compound and have a varying number of pinnae.
The number of pinnae per leaf may be 3, 5, 6, 7 or 8. Frequently it is four as seen in M.quadrifolia (Fig.164). The leaves show circinate vernation. The pinnae vary in their shape. They may be obovate, obcuneate or elliptical. They may or may not be lobed. The margin of the pinnae may be smooth or dentate (M.minuta). The venation is of the closed reticulate type. The pinnae exhibit sleeping movements.
Roots are borne on the under-surface of the rhizome at the nodal region. But is is not uncommon to find roots emerging from the intermodal region. Roots develop in acropetalous succession (youngest near the growing tip of the rhizome).
The plant bears reproductive structures which are very special to Marseilles. These are called sporocarps. The sporocarps have a stalk called the peduncle or the pedicel. The peduncle may be attached to the stem or the petiole. The sporocarps are bean shaped and bisporangiate.
Internal Structure:
1. Rhizome:
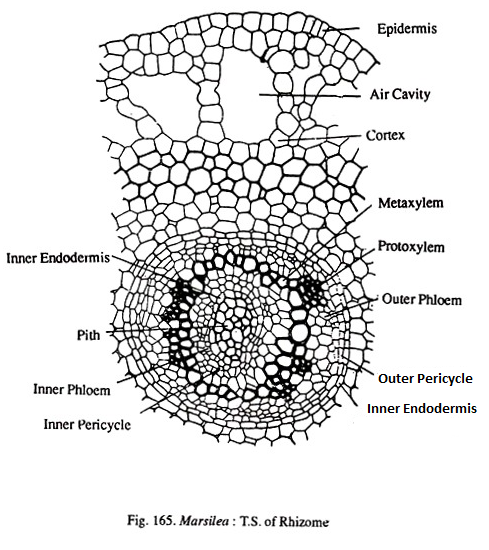
A transection shows the usual three regions viz., epidermis, cortex and central stele (Fig.165). Epidermis is single layered, continuous and lacks stomata. The cortex has many regions. The outermost cortex consists of compactly arranged parenchyma cells. Next to this is the aerenchymatous cortex.
ADVERTISEMENTS:
This consists of broad air spaces separated by parenchyamatous partitioins. Internal to the aerenchyma is the sclerotic zone having one or two layers of thick walled cells. The innermost region of the cortex is again parenchymatous. In this region may be found a few tannin cells.
The central vasculature is an amphiphloic solenostele. As is typical to such steles there is an outer endodermis, outer pericycle, outer phloem, xylem, inner phloem, inner pericycle and inner endodermis. The composition of the pith varies with the habitat.
It is sclerotic in terrestrial species but parenchymatous in aquatic species. In the xylem ring there may (M.vestita) or may not be (M.quadrifolia) a distinction between proto and metaxylem. When a distinction exists there are many exarch protoxylem points. The xylem may be mesarch in Maegyptiaca. Leaf traces depart from the vascular cylinder to provide the leaf.
2. Leaf:
ADVERTISEMENTS:
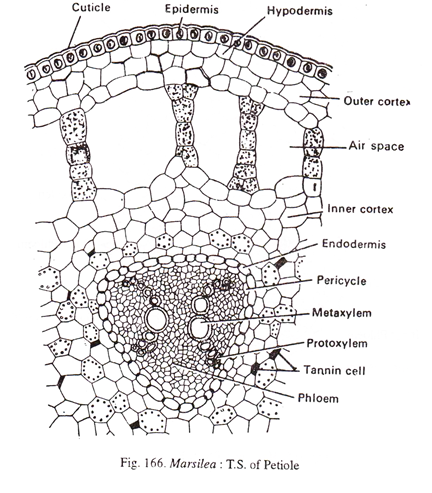
The petiole (Fig.166) has an outermost epidermis. The cortex has two zones viz., an outer aerenchymatous and an inner parenchymatous zone. The inner zone is rich in starch and tannin. The vasculature consists of a ‘V’ shaped xylem with the two arms slightly bent outwards. Sometimes the two arms may be separated at the base also. The xylem is surrounded by phloem, pericycle and endodermis.
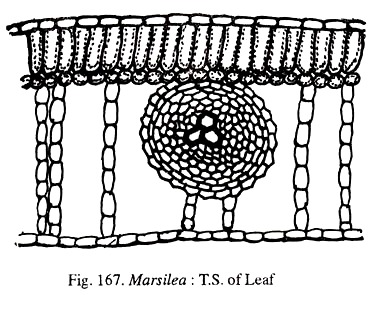
The lamina is bifacial (Fig.167). It has an upper and lower epidermis bounding the mesophyll. Leaves may be epistomatic (aquatic) or amphistomatic (terrestrial). The mesophyll shows clearly defined palisade and spongy parenchyma. Vascular bundles are concentric with the centrally situated xylem surrounded by phloem.
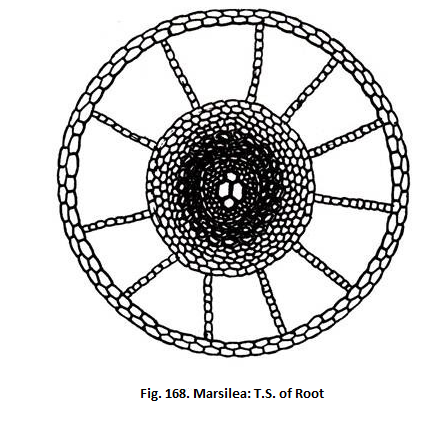
3. Root:
ADVERTISEMENTS:
Anatomically the root shows a single layered epidermis, a three zoned cortex and a central protostele (Fig.168). The cortex has an outer aerenchymatous, a middle parenchymatous and an inner sclerotic zone. Stele is bounded by an endodermis and pericycle. Xylem consists of two large metaxylem tracheids and two protoxylem tracheids. It is exarch and diarch. Vessels have been reported in M.quadriofolia. M.drummondii and M.hirsuta.
Reproduction:
ADVERTISEMENTS:
The sporophyte reproduces by vegetative propagation as well as by spore production. Vegetative propagation is brought about by tubers (M. hirsuta). These are small, condensed side branches having leaf primordia. The tubers persist even after a plant dies during unfavourable conditions and on the availability of favourable conditions, develop into a new plant.
Spore Producing Organs:
As has already been pointed out, the spore bearing structures, are called sporocarps. Each sporocarp has a short stalk pedicel. The mode of attachment of the pedicel to the plant is varied. In M. polycarpa and M. subangulata the pedicels are directly attached to the petiole in a linear sequence.
In M.quadrifolia pedicels of many sporocarps join together and have a common stalk joined to the petiole. In M.vestita a single pedicel is attached to the base of the petiole. The sporocarp is bisporangiate and is usually light green in colour. At the point of attachment of the pedicel to the body of the sporocarp there are one to two teeth like projections called ‘tubercles’.
Development of the Sporocarp:
ADVERTISEMENTS:
Sporacarp development has been studied in detail by Johnson (1898). The sporocarp originates early in the ontogeny of the leaf. In a young leaf one of the transversely placed apical cell gives rise to the sporocarp long before the appearance of the lamina. In a young growing sporocarp, the tip enlarges and is directed horizontally.
With the enlargement of the tip, two rows of soral mother cells appear on the ventral side. These give rise to two rows of son. When sori are developing, the marginal cells withdraw and form spaces, called soral canals. The soral canal is lined at its inner face by a tissue, called inducium. Each sorus consists of a band of fertile tissue, called receptacle. It is in this, the sporangial initials arise.
Development and Structure of Sporangia:
Marsilea is heterosporous. The sporocarp bears both the types of sporangia. In fact each sorus produces both microsporangia and mega-sporangia. The sporangial initials develop along the top and sides of the receptacular tissue in the sorus. The initials that appear first the top of the sorus develop into mega-sporangia, while those towards the sides develop into microsporangia. The soral nature may be said to be of the gradate type.
Early stages of development are similar in both micro and mega-sporangia. The differentiation sets in only at the spore mother cell stage. One of the superficial cells on the sorus functions as the sporangial initial. This divides periclinally to form an outer cell and a lower cell.
ADVERTISEMENTS:
The inner cell does not contribute to any part of the sporangium. The outer cell cuts off a tetrahedral apical cell by three intersecting divisions. Segments that are cut off from the three sides develop into stalk and basal portion of the jacket.
The apical cell, then divides periclinally to form an outer jacket initial and an inner primary archesporial cell. The jacket initial (by anticlinal divisions) builds up the single layered jacket. The primary archesporial cell on each of its side cuts off tapetal initials.
The tapetal initials develop a two to three layered tapetum. The central cell (archesporial cell) is now called the sporogenous cell and divides repeatedly to form 8-16 spore mother cells. These divide meiotically to form 32-64 spores.
Initially, the number of spores appears to be the same in both micro and mega-sporangia. Subsequently, in a sporangium that is to become a mega-sporangium all spores except one disintegrate. The disintegrating spores add to the periplasmodiuim formed by the tapetum.
The surviving megaspore enrages and almost fills the sporangial cavity. In a sporangiuim that is to become a micro sporangium all the spores are functional. There is no special dehiscence mechanism in both the types of sporangia.
Structure of the Mature Sporocarp:
The sporocarp is made up of two valves which can be split open along the dorsiventral plane so as to reveal the internal structure. The wall of the sporocarp is very thick. Adhering to the wall is a gelatinous ring that extends in the sporocarp cavity. There are two rows of sori, one row corresponding to one value.
The number of sori per row ranges from 2-20. The sori are arranged approximately parallel to the pedicel of the sporocarp. Each sorus is encased in a thin inducium and has a row of mega-sporangia on top and two rows of microsporangia towards the sides. In M. rajasthanensis, sometimes the sporocarps may have only microporangia.
Vasculature in the Sporocarp:
The sporocarp is supplied by a single vascular trace called the Dorsal median bundle (DMB). At the region of the tubercle this forks into two and the branches supply the two valves. From each branch of the DMB a number of laterals or commissural are given out, one corresponding to each sorus.
The laterals also fork halfway and the two forks run on either side of the sorus. From the point of forking of the lateral, another bundle (placental bundle) starts and enters into the receptacle where it also forks. The ends of the form of the laterals are not free. They fuse with those of preceding and succeeding forks forming a closed network.
Detailed Internal Structure of the Sporocarp:
Sections cut at different planes clearly reveal the internal structure.
The following are the three planes of section:
1. Vertical longitudinal section or section cut parallel to the flat surface of the sporocarp (Fig.172).
2. Horizontal section or section perpendicular to the pedicel (Fig.173).
3. Vertical transverse section or section perpendicular to the flat surface of the sporocarp (Fig. 174).
Vertical longitudinal section:
The section shows a wall made up of three layers of cells. The outermost is epidermis which has stomata and two hypodermal layers. The hypodermal cells are columnar. Next to the wall is the continuous gelatinous ring.
Sori are cut lengthwise. Sori of only one valve can be seen. If the section is slightly superficial only microsporangia (two rows) are seen (Fig.172) and, if the section is deeper only mega-sporangia (one row) are seen. An inducium envelops all the sori.
Horizontal section:
In a horizontal section, the pedicel will be cut transversely. It is in this section that the sori of both the valves can be seen. The wall of the sporocarp consists of three layers of cells. Its structure is same as in vertical longitudinal section.
The gelatinous ring is cut transversely and can be seen in the form of two patches at either end of the section. All the sori are cut transversely, so also the indicial covering and the lateral bundles which can be seen at the base of the sorus. In each sorus can be seen a terminal mega-sporangium and two lateral microsporangia.
Vertical transverse section:
In this section both the valves are cut. The wall consists of the usual three layers. The gelationous ring is cut transversely and is present in the form of two patches at either end of the section. Sori are cut length wise and each sorus represents a valve. Inducia are attached to this. A slightly superficial section shows only microsporangia and a deeper section shows a row of mega-sporangia with one or two microsporangia at either end of the sorus.
Dehiscence of the sporocarp and liberation of the sori: There does not seem to be a natural dehiscence mechanism brought about by the inherent force. The wall is very thick and unless probably injured by external agents, the sporocarp may remain unopened for a long time. There are reports of viable sporocarps as old as 30-35 years.
When there is an injury, the sporocarp absorbs water and splits open along the ventral suture. The gelatinous ring first protrudes out a little (Fig.175). A break occurs in the ring and the ring extends out like a long gelatinous band carrying with it, the sori.
The gelatinous band is called the sorophore since it looks like a stalk bearing the sori. The sori break their connections with the sorophore so also the sporangia with the sori. Ultimately the sporangial wall ruptures liberating the spores.
Gametophyte of Marsilea:
Marsilea is heterosporous. As such there are two types of gametophytes. Both the male and female gametophytes are endosporic.
Structure of the microspore and development of the male gametophyte:
Microspores are generally globose. The diameter of the spore ranges from 0.06 to 0.075 mm. There are two wall layers. There is a centrally situated nucleus surrounded by starch rich cytoplasm.
The germination of the microspore is very rapid and the entire development is completed within about 10-12 hours resulting in the release of antherozoids. The nucleus of the microspore migrates to a side where it undergoes a division (wall 1-1) to cut off a small prothallial cell and a large primary antheridial cell (Fig.176a).
In some cases, (M.elata) a second prothallial cell may be formed. The primary antheridial cell divides by an oblique or diagonal wall to form two antheridial cells (Fig. 176b). Each of which forms an antheridium. Each antheridial cell cuts off a periclinal cell to form a central cell and a jacket cell (Fig.176c).
The central cell cuts off a small sterile cell and a large cell. The sterile cell forms the second jacket cell. The large cell divides periclinally and forms the third jacket cell and a central primary androgonial cell. The sequence of wall formation is given in Fig. 176.
At this stage the antheridium has one prothallial cell, two primary androgonial cells and six jacket cells. Each primary androgonial cell gives rise to 16 antherozoids (totally there will be 32 in the gametophyte) which are cork screw shaped and multi-flagellate (Fig. 176j-176k).
Since the androcytes are in two groups (Fig.176e, 176h) separated by the sterile cells, it has been assumed that there are two antheridia in the gametophyte. At maturity the jacket cells disintegrate liberating the antherozoids.
Structure of the Megaspore and Development of the Female Gametophyte:
The megaspore is ellipsoidal to oval in shape and has a small papilla protruding at one end (Fig.177b). Size varies from 0.41 x 0.36 to 0.8 x 05 mm.
According to Chrysler and Johnson (1939), four layers are recognizable in the megaspore. These are:
(a) Endospore, this is continuous under the papilla;
(b) Inner layer of epispore not seen under the papilla;
(c) Prismatic layer of epispore, this is also continuous under the papilla and
(d) Outer epispore, continuous under the papilla.
The single nucleus of the megaspore is situated in the papilla region. The basal part of the megaspore is rich in reserve food materials like, starch, oil and albuminous granules.
Detailed account of the development of the female gametophyte has been studied in M.vestita by Campbell (1892). The foregoing account is based mainly on his observations.
As in the male gametophyte, in the female gametophyte also the development is rapid and it is completed in about 12-15 hours.
The lust division is transverse and it cuts off a small cell limited to the papilla region and a huge basal cell occupying the remainder of the cavity. The entire gametophyte is derived from the papilla cell. The basal cell does not divide further.
It increases its reserve food content. Its only function is nutrition of the developing gametophyte and embryo. Subsequent divisions in the papilla cell result in the formation of a central cell surrounded by three peripheral cells.
This cuts off a basal cell and functions as the archegonial initial. The remaining cells divide transversely as well as vertically to build up a small amount of vegetative tissue (Fig.177c). The archegonial initial divides periclinally to form an upper primary cover cell and a lower central cell. The primary cover cell divides to form a neck of two tiers of four cells each.
The central cell divides to form a primary canal cell and a primary venter cell. The primary canal cell may or may not divide. Primary venter cell divides to form a venter canal cell and an egg cell.
A mature female gametophyte has a cap of tissue enclosing the single acrheogonium and a basal unicellular portion. The nucleus in the basal nutritive portion of the gametophyte may divide amiotically as in M.drum- mondii. The female gametophyte is surrounded by a gelatinous layer which has a funnel shaped opening above (Fig. 177a) to receive the antherozoids.
Embryogeny:
Like in the development of gametophytes, embryogeny is also very rapid and the first leaf projects out within 2-4 days after fertilization. The first division of the zygote seems to be influenced by gravity. It is parallel to the long axis of the archegonium and since the female gametophyte is prone, zygote forms an upper cell and lower cell. The upper cell gives rise to stem and leaf and the lower cell gives rise to foot and root.
Quadrant divisions follow next, and they are followed by octant walls (Fig.178). When the embryo is developing, a protective sheath called calyptra develops from the gametophyte. Root grows vertically down and establishes the sporeling in the soil. Earliest leaves are simple and do now show circinate vernation.
Parthenogenesis:
Strassburger (1907) has reported parthenogenetic development of diploid eggs (which are apparently produced by the failure of meiosis during mega-sporogenesis) in M.drummondii. Chromosome number: In M. minuta and M. brachypus n = 20.
Morphology of the sporocarp:
There are two divergent views regarding the morphological nature of the sporocarp. The first, leaf segment or laminar hypothesis equates the sporocarp with one or more pinnae of the leaf. This theory finds greatest number of supporters like Bower (1889), Goebel (1891) etc.
The second, petiolar hypothesis holds that the sporocarp is equal to the entire leaf and the sporangia are derived from cells which should have given rise to the lamina. The lone supporter of this theory is Johnson (1898, 1933).
Laminar Hypothesis:
There is apparently no agreement among the supporters of this theory as to the number of pinnae involved in sporocarp formation or the mode of their transformation into the sporocarp. The various views have been summarise by Parihar (1973).
A few of these are given below:
(a) Goebel (1891) regards the sporocarp to be equivalent to one or more pinnae.
(b) According to Russow (1872) and Busgen (1890), the sporocarp is made up of two pinnae with their ventral surfaces (with sori) facing each other.
(c) Campbell (1940), regards the sporocarp to be a folded pinnate leaf developing from the rachis of the fertile leaf.
(d) Bower (1889), believes the sporocarp to possess a rachis bearing two rows of pinnules folded up.
(e) According to Eames (1936), the sporocarp is a modified tip of the leaf whose margins bend downwards. Side by side a wing like shoulder develops protecting the sori.
(f) Smith (1955), interprets the sporocarp as having a pinna with two sub marginal sori. The two edges of the pinna fold down, meet each other and completely enclose the sori.
(g) According to Puri and Garg (1953), the sporocarp is a single pinna having lobes or pinnules as many in number as there are sori. The two rows of the pinnules fold on the midrib of the pinna to produce the sporocarp.
(h) Gupta (1962), holds the sporocarp to be a single pinna with many lobes.
There are quite a good number of evidences to support the leaf segment hypothesis. The occurrence of abnormal, sporocarp like leaves in M. hirsuta give credence to the laminar view. There is sufficient evidence by, the vascular supply also to support this hypothesis.
Petiolar Hypothesis:
Johnson (1933) is the lone crusader for this theory. He holds the sporocarp to be an equivalent of an entire leaf (lamina + petiole) because the apical cell that produces the leaf and the sporocarp are (cell with two cutting faces) alike, whereas the lamina of the normal leaf is produced by many, five sided marginal cells. As Johnson (1933) opines, if the sporocarp is a modified lamina it should also be produced from many marginal cells.
The fact that the sporocarp is derived from a single apical cell (as in leaf) shows that it is an entire leaf. He observes “according to our present knowledge we may consider the capsule as the swollen end of a petiole in which the marginal cells are devoted to the formation of sporangia instead of a lamina”.
Phylogeny of Marsilea:
The Marsileaceae have a plant body that has deviated to a great extent from other ferns. The adoption of heterospory is an outstanding character. The formation of sporocarp that has no parallel in any other fern group is surely related to its habitat.
Many people have put together Marsileales and Salviniales in a group called Hydropteridinae. Eames (1964) however, feels that Hydropteridinae is an unnatural assemblage because the members are not fundamentally different from other fern groups.
Marsileaceae seems to be phylogentically related to Schizaeaceae having been derived from it. This view seems to be supported by the arrangement of pinnae in Schizaeaceae and Marsileaceae.