ADVERTISEMENTS:
In this article we will discuss about the composition and structure of proteins.
Composition of Proteins:
Proteins are large molecules consisting of many amino-acids connected by “peptide linkages”.
Peptide bond is produced when carboxyl radical  of one amino acid reacts with the amino (-NH2) group of the other amino acid. The basic structural formula of amino acids is shown in Fig. 4.1.
of one amino acid reacts with the amino (-NH2) group of the other amino acid. The basic structural formula of amino acids is shown in Fig. 4.1.
It consists of one alpha (a) carbon atom that is associated with an amino group (-NH2) with a potential (+) charge, a carboxyl group  with a (-) charge, a hydrogen atom and a side chain “R” that varies in the different amino acids.
with a (-) charge, a hydrogen atom and a side chain “R” that varies in the different amino acids.
There are usually 20 amino acids found in proteins (for structural formulae of the amino acids, any book of biochemistry may be consulted). These twenty amino-acids are divided into 7 groups (Table 4.1). All the 20 amino acids need not be present in a given protein.
The side chains (R) of amino acids are responsible for the different properties, such as, water solubility, interaction with other amino acids etc. of the amino acids. The amino acids possessing -CH3 group are much less soluble in water and they are called “hydrophobic” amino acids, e.g., leucine, isoleucine, valine.
The amino acids that are water soluble are called “hydrophilic” amino-acids, e.g., lysine (+ charge) and aspartic acid (-charge). The sulfhydryl group (-SH) of cysteine can interact with the -SH group of other cysteine in the protein chain to make a disulfide linkage (S-S). The H atoms of hydroxyl group (-OH) or carboxyl group 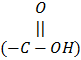 of the “R ‘ chain can make hydrogen bonding with other amino acids in the protein chain. The bonds are required for stabilizing the structure of protein molecules.
of the “R ‘ chain can make hydrogen bonding with other amino acids in the protein chain. The bonds are required for stabilizing the structure of protein molecules.
Structure of Proteins:
The protein molecule containing a single polypeptide chain (monomeric protein) can take primary, secondary and tertiary structures. The protein composed of two or more polypeptide chains (multimeric proteins) can take one more degree of conformation, the “quaternary structure”.
Primary Structure:
Proteins are long polypeptide chains. One end of their chain contains the free amino group (-NH2), while the other end contains the free carboxyl group  . The amino acids of the chain are linked with peptide bonds
. The amino acids of the chain are linked with peptide bonds  .
.
Number of amino acids and their sequence varies in different types of proteins. Because the sequence and number of amino acids in the proteins are determined by the information contained in the gene encoding them, the primary structure of proteins can be compared with the base sequence of the concerned nucleic acid as follows.
(1) Proteins are polypeptides made of linked amino acids, whereas nucleic acids are polynucleotides made of ribonucleotides (RNA) and deoxyribonucleotides (DNA).
(2) Proteins contain -NH2 group at one end and  group at the other end, whereas nucleic acids contain phosphoric acid at one end (5′-end) and -OH group at the other end (3′-end).
group at the other end, whereas nucleic acids contain phosphoric acid at one end (5′-end) and -OH group at the other end (3′-end).
(3) Amino acids are linked by peptide bonds  in proteins whereas nucleotides are linked by phosphodiester bonds (-O-P-O) in nucleic acids.
in proteins whereas nucleotides are linked by phosphodiester bonds (-O-P-O) in nucleic acids.
Secondary Structure:
The secondary structure of proteins occurs due to the formation of non-covalent H-bonds between -NH and -CO groups of the amino acids which are very close to each other. Most of the proteins are coiled into a right-handed helix called the “alpha helix”. Other types of secondary structure are “beta pleated sheet” where polypeptide chains lie side by side, stabilized by H-bonds.
ADVERTISEMENTS:
Tertiary Stricture:
Polypeptide chain bends and folds at various places to produce some spherical conformation. The structure is stabilized by side chain of amino acids. The sites of specificity of enzymes are formed due to tertiary structure.
Quaternary Structure:
Some proteins exist as association of two or more polypeptide chains. Differed polypeptide chains are bound together by non-covalent and occasionally by covalent bonds. For example, a complete human hemoglobin molecule consists of four individual polypeptide chains, two identical alpha (α) chains and two identical beta (β) chains.
ADVERTISEMENTS:
The individual a chains consist of 140 amino acids and the individual β chains consist of 146 amino acids. The core enzyme of RNA polymerase is composed of 5 polypeptide chains, β’ βα2ω.
Protein synthesis occurs under the direction of information stored in DNA as “codes”. Different kinds of RNAs, such as ribosomal RNA, transfer RNA and messenger RNA are produced on DNA template through the process of “transcription”.
Protein synthesis occurs on ribosomes where tRNA, brings amino acids to form polypeptide, according to the “codon’ in the mRNA thus the message from DNA is translated into a protein. Different components of protein synthesis are described in the following sections.

