ADVERTISEMENTS:
Read this article to learn about the nine things of protein engineering.
1. Increasing the Stability and Biological Activity of Proteins:
By increasing the half-lives or thermo stability of enzymes/proteins, their industrial applications or therapeutic uses can be more appropriately met. Some of the approaches for producing proteins with enhanced stability are described below.
2. Addition of Disulfide Bonds:
ADVERTISEMENTS:
Significant increase in the thermo stability of enzymes is observed by adding disulfide bonds. However, the additional disulfide bonds should not interfere with the normal enzyme function. In general, the new protein with added disulfide bonds does not readily unfold at high temperatures, and further it is resistant to denaturation at non-physiological conditions (high pH, presence of organic solvents). These characteristics are particularly important for industrial applications of certain enzymes.
T4 Lysozyme:
This an enzyme of bacteriophage T4. Good success has been achieved in introducing disulfide bonds in T4 lysozyme. This was done by changing two, four or six amino acids (in close proximity) to cysteine residues to respectively form one, two or three disulfide bonds.
In the wild type (native) T4 lysozyme, there are two cysteine residues which however are not held together by a disulfide bond. By oligonucleotide-directed mutagenesis, cysteine residues created disulfide bonds between positions (numbered form N-terminal end) 3 and 97, 9 and 164, and 21 and 142 of the enzyme.
ADVERTISEMENTS:
Introduction of disulfide bonds increases the folded structure and thermo stability of the enzyme. Thus, T4 lysozyme with three disulfide bonds is very stable with good biological activity. (Note: T4 lysozyme has no industrial or therapeutic applications. It is discussed here due to the success achieved in protein engineering).
Xylanase:
This is an enzyme used in the industry for manufacture of paper from wood pulp. Xylanase has to be catalytically active at high temperature. Introduction of disulfide bonds (one, two or three) makes it thermo-stable, and substantially improves its functional efficiency.
3. Changing Asparagine to Other Amino Acids:
At high temperature, the amino acids asparagine and also glutamine are likely to undergo deamidation (releasing ammonia) to respectively form aspartic acid and glutamic acid. These alterations are often associated with changes in the protein folding and loss of biological activity.
Triose phosphate isomerase:
This is a dimeric enzyme with identical subunits, each one having two asparagine residues which are thermo-sensitive (undergo deamidation). Oligonucleotide-directed mutagenesis was used to introduce threonine or isoleucine in place of asparagine. The new enzyme was found to be thermo-stable. On the other hand, when the asparagine residues were replaced by aspartic acid, the enzyme was unstable even at low temperature, with reduced activity.
4. Reducing the Free Sulfhydryl Groups:
Sometimes, the presence of free sulfhydryl groups (contributed by cysteine residues) in more numbers may be responsible for the low activity of the protein. In such a case, the protein or enzyme stability and its activity can be increased by reducing the number of sulfhydryl groups.
ADVERTISEMENTS:
Human β-interferon:
The antiviral activity of human β-interferon (IFN β) produced in E. coli by genetic engineering was found to be only 10% of the original glycosylated form. Further, IFN β was found to exist as dimers and oligomers which are almost inactive. In fact, the cysteine residues were involved in intermolecular disulfide bonding resulting in the formation of dimers and oligomers.
This happens in E. coli cells and not in human cells. This problem was successfully overcome by replacing cysteine residues by serine. It may be noted that the structures of these two amino acids are similar except that serine has oxygen in place of sulfur in cysteine. Consequently, introduction of serine in place of cysteine reduces free sulfhydryl groups. By the above process, IFN β with increased stability and good biological activity can be produced. Increased stability is in fact required for storage and therapeutic use of proteins such as interferon’s.
5. Single Amino Acid Changes:
ADVERTISEMENTS:
Some of the recombinant proteins can be improved in their stability and biology activity by a second generation variants. These have been frequently achieved by a single amino acid change.
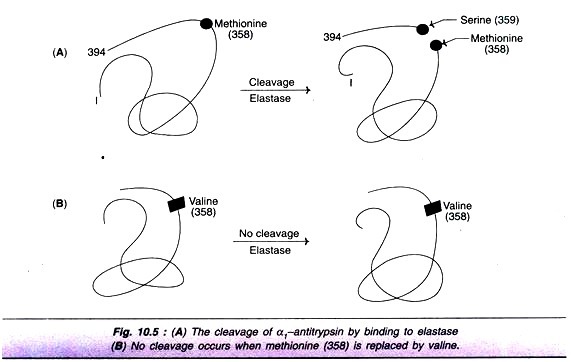
α1-Antitrypsin:
α1-Antitrypsin inhibits the action of neutrophil elastase (elastase is an enzyme that damages the lung tissues, often resulting in emphysema i.e. abnormal distension of lungs by air), α1,-Antitrypsin binds to elastase and prevents its action. In this process, α1,-antitrypsin gets cleaved between serine and methionine . The free methionine is oxidized to methionine sulfoxide, making α1-antitrypsin a poor inhibitor of elastase.
By replacing methionine at by valine, an oxidative-resistant variant of α1-antitrypsin has been created (Fig. 10.5). This new enzyme is particularly important in treating patients with genetic deficiency of α1,-antitrypsin.
Insulin:
In the neutral solution, therapeutic insulin is present mostly as zinc—containing hexamer. By introducing single amino acid substitutions, insulin’s were found to be in monomeric state with good stability and biological activity.
ADVERTISEMENTS:
Tissue plasminogen activator (tPA):
Tissue plasminogen activator is therapeutically used to lyse the blood clots that cause myocardial infarction. Due to its shorter half-life (around 5 minutes), tPA has to be repeatedly administered. By replacing asparagine residue with glutamine, the half-lite of tPA can be substantially increased. This is due to the fact that glutamine is less glycosylated than asparagine and this makes a difference in the half-life of tPA.
Hirudin:
Hirudin is a protein secreted by leech salivary gland, and is a strong thrombin inhibitor (i.e., acts as an anticoagulant). By replacing asparagine with lysine, the potency of hirudin can be increased several-fold.
Dihydrofolate reductase:
The enzyme dihydrofolate reductase (DHFR) catalyses the conversion of 7, 8-dihydrofolate to 5, 6, 7, 8-tetrahydrofolate. The latter coenzyme is closely involved in one carbon metabolism, which ultimately results in the synthesis of nucleic acids and amino acids. The inhibition of DHFR (conventionally by folate analogues such as methotrexate) will restrict the growth of tumor cells. Therefore the enzyme DHFR has some therapeutic applications. By employing site-directed mutagenesis, replacement of glycine by alanine was found to produce DHFR which is completely inactive.
ADVERTISEMENTS:
T4 lysozyme:
Replacement of glycine by any other amino acid in the protein structure, in general, decreases the stability. On the other hand, pro-line residues increase protein stability. In T4 lysozyme, substitution of glycine by alanine, and alanine by pro-line are found to increase the enzyme stability.
6. Improving Kinetic Properties of Enzymes:
It is possible to improve the functional activities of enzymes, by improving their kinetic properties (Km, specificity etc.) through oligonucleotide- directed mutagenesis. This is particularly required for enzymes with industrial and therapeutic applications.
Subtilisin:
Subtilisin is a major industrial enzyme secreted by Gram-positive bacteria (Bacillus species). It is a serine protease very widely used as an enzyme detergent (cleaning agent in laundries). However, the large scale industrial use of native subtilisin is restricted due to the oxidative inactivation of this enzyme. This occurs in a manner analogous to that already discussed for α1-antitrypsin.
The reason being that methionine lying close to the active site gets oxidized, making the enzyme inactive. Logically, replacement of methionine by other amino acids will solve the problem. This has been done, and in fact, all the 19 other amino acids have been tried as substitutes for methionine, with varying success.
ADVERTISEMENTS:
Subtilisin is an enzyme that has been extensively exploited for genetic manipulations over the past 15 years. The result is that about 50% of the native amino acids of this enzyme have been changed by in vitro mutagenesis. And almost every property of subtilisin has been altered. These include its stability, substrate specificity, thermal and alkaline inactivation.
Modifying metal requirement of subtilisin:
The enzyme subtilisin binds to calcium and gets stabilized. But in many industries where subtilisin is used, metal-chelating agents are also employed. They bind to calcium and the enzyme becomes inactive. This problem can be solved by oligonucleotide-directed mutagenesis.
The nucleotide sequence in the DNA encoding for the amino acids that bind to calcium is removed. Then, several other amino acids are modified in subtilisin by trial and error method. In fact, success has been achieved in creating calcium-free subtilisin with good stability and activity.
Asparaginase:
This is an enzyme used in the control of leukemia (uncontrolled growth of white blood cells). Intravenous administration of asparaginase cleaves asparagine to aspartate (the reduced availability of asparagine restricts cell proliferation). Surprisingly, the asparaginases from different sources exhibited differences in their effectiveness to control leukemia.
This is due to the variations in the kinetic properties. Thus, the asparaginase with a low Km value (i.e., with high affinity for asparagine hence more breakdown) has to be selected for therapeutic use in the control of leukemia.
ADVERTISEMENTS:
Tyrosyl t-RNA synthetase:
The enzyme tyrosyl t-RNA synthetase from Bacillus stearothermophilus has been modified with regard to substrate binding i.e., Km value. This enzyme catalyses the two reactions given below to finally give tyrosine t-RNA.
Tyrosine + ATP→ Tyrosyladenylate + PPi
Tyrosyladenylate + t-RNA → Tyrosine t-RNA + AMP
Replacement of threonine by either alanine or proline, variants of tyrosyl t-RNA synthetase have been produced. Alanine variant has a twofold binding affinity (low Km) for ATP. And for proline variant ATP binds about 100-fold more tightly (very low Km).
Restriction endonucleases:
The protein engineering studies can be successfully employed for modifying enzyme specificities. This is what has been achieved in oligonucleotide-directed mutagenesis with respect to restriction endonucleases. So far, about 2500 restriction endonucleases are known. However, since most of these enzymes recognize the same sequence on the DNA for their action, there are only about 200 different recognition sites.
Thus there is an overlap in the recognition sites of several restriction enzymes. As such, there are two types of restriction endonucleases—the frequent cutters which recognize a sequence of 4-6 bp and rare cutters recognizing a sequence above 8 bp. The rare cutters are more useful for producing large DNA fragments.
By using protein engineering technique, the existing restriction endonucleases have been suitably modified to produce rare cutters.
7. Protein Engineering By Use of Gene Families:
The recent development in protein engineering is DNA shuffling, also known as molecular breeding (developed by Nesse in 2000). This method can be applied to a protein if it belongs to a known protein family. The technique primarily involves isolation of genes from each species, and then creation of hybrids in different combinations. While applying this approach to subtilisin, genes from 26 species were mixed to create a library. Among these, 4 enzymes were found to have improved properties.
8. Protein Engineering Through Chemical Modifications:
Although with limited success, chemical modifications of proteins have been attempted to increase the stability of proteins to high temperature and organic solvents. The amino acid lysine residues can be cross-linked by use of chemical linkers.
The most extensively used protein cross linker is glutaraldehyde. It stabilizes the proteins in solutions. By using glutaraldehyde, certain proteins (hemoglobin, insulin, phosphofructokinase, lactate dehydrogenase) have been stabilized.
9. Protein Engineering—An Ever Expanding Field:
Selected examples of protein engineering are described above only to highlight the scope of protein engineering. The techniques of enzyme engineering are rapidly expanding and several newer developments are in the offing to develop proteins/enzymes for industrial and therapeutic purposes. The question before biotechnologists specialized in protein engineering is not ‘what modifications are possible’, but ‘how do I achieve the modifications I wish to make’.