ADVERTISEMENTS:
This article throws light upon the five stages of protein biosynthesis. The five stages are: (1) Requirement of the Components (2) Activation of Amino Acids (3) Protein Synthesis Proper (4) Chaperones and Protein Folding and (5) Post-Translational Modifications of Proteins.
The protein synthesis which involves the translation of nucleotide base sequence of mRNA into the language of amino acid sequence may be divided into the following stages for the convenience of understanding.
1. Requirement of the components
ADVERTISEMENTS:
2. Activation of amino acids
3. Protein synthesis proper
4. Chaperones and protein folding
5. Post-translational modifications.
Stage # 1. Requirement of the Components:
The protein synthesis may be considered as a biochemical factory operating on the ribosomes. As a factory is dependent on the supply of raw materials to give a final product, the protein synthesis also requires many components.
a. Amino Acids:
ADVERTISEMENTS:
Proteins are polymers of amino acids. Of the 20 amino acids found in protein structure, half of them (10) can be synthesized by man. About 10 essential amino acids have to be provided through the diet. Protein synthesis can occur only when all the amino acids needed for a particular protein are available.
If there is a deficiency in the dietary supply of any one of the essential amino acids, the translation stops. It is, therefore, necessary that a regular dietary supply of essential amino acids, in sufficient quantities, is maintained, as it is a prerequisite for protein synthesis. As regards prokaryotes, there is no requirement of amino acids, since all the 20 are synthesized from the inorganic components.
b. Ribosomes:
The functionally active ribosomes are the centres or factories for protein synthesis. Ribosomes may also be considered as workbenches of translation. Ribosomes are huge complex structures (70S for prokaryotes and 80S for eukaryotes) of proteins and ribosomal RNAs. Each ribosome consists of two subunits—one big and one small. The functional ribosome has two sites— A site and P site. Each site covers both the subunits.
A site is for binding of aminoacyl tRNA and P site is for binding peptidyl tRNA, during the course of translation. Some authors consider A site as acceptor site and P site as donor site. In case of eukaryotes, there is another site called exist site or E site. Thus, eukaryotes contain three sites (A, P and E) on the ribosomes.
The ribosomes are located in the cytosomal fraction of the cell. They are found in association with rough endoplasmic reticulum (RER) to form clusters RER—ribosomes, where the protein synthesis occurs. The term polyribosome (polysome) is used when several ribosomes simultaneously translate on a single mRNA (Fig. 4.15).
c. Messenger RNA (mRNA):
The specific information required for the synthesis of a given protein is present on the mRNA. The DNA has passed on the genetic information in the form of codons to mRNA to translate into a protein sequence.
d. Transfer RNAs (tRNAs):
They carry the amino acids, and hand them over to the growing peptide chain. The amino acid is covalently bound to tRNA at the 3′-end. Each tRNA has a three nucleotide base sequence—the anticodon, which is responsible to recognize the codon (complementary bases) of mRNA for protein synthesis. In man, there are about 50 different tRNAs whereas in bacteria around 40 tRNAs are found. Some amino acids (particularly those with multiple codons) have more than one tRNA.
e. Energy Sources:
ADVERTISEMENTS:
Both ATP and GTP are required for the supply of energy in protein synthesis. Some of the reactions involve the breakdown of ATP or GTP, respectively, to AMP and GMP with the liberation of pyrophosphate. Each one of these reactions consumes two high energy phosphates (equivalent to 2 ATP).
f. Protein Factors:
The process of translation involves a number of protein factors. These are needed for initiation, elongation and termination of protein synthesis. The protein factors are more complex in eukaryotes compared to prokaryotes.
Stage # 2. Activation of Amino Acids:
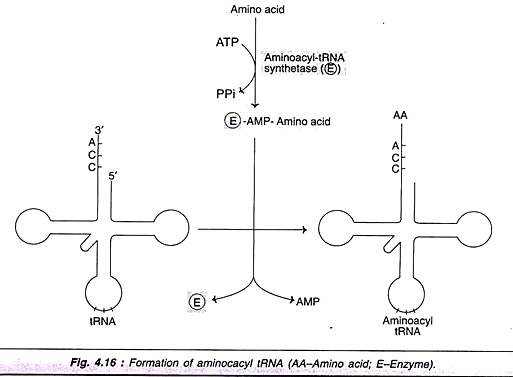
Amino acids are activated and attached to tRNAs in a two-step reaction. A group of enzymes—namely aminoacyl tRNA synthetases—are required for this process. These enzymes are highly specific for the amino acid and the corresponding tRNA. The amino acid is first attached to the enzyme utilizing ATP to form enzyme-AMP-amino acid complex. The amino acid is then transferred to the 3′ end of the tRNA to form aminoacyl tRNA (Fig. 4.16).
Stage # 3. Protein Synthesis Proper:
The protein or polypeptide synthesis occurs on the ribosomes (rather polyribosomes). The mRNA is read in the direction and the polypeptide synthesis proceeds from N-terminal end to C-terminal end. Translation is directional and collinear with mRNA. The prokaryotic mRNAs are polycistronic, since a single mRNA has many coding regions that code for different polypeptides. In contrast, eukaryotic mRNA is monocistronic, since it codes for a single polypeptide.
ADVERTISEMENTS:
In case of prokaryotes, translation commences before the transcription of the gene is completed. Thus, simultaneous transcription and translation are possible. This is not so in case of eukaryotic organisms since transcription occurs in the nucleus whereas translation takes place in the cytosol. Further, the primary transcript (hnRNA) formed from DNA has to undergo several modifications to generate functional mRNA.
Protein synthesis is comparatively simple in case of prokaryotes compared to eukaryotes. Further, many steps in eukaryotic translation were not understood for quite some time. For these reasons, majority of the textbooks earlier used to describe translation in prokaryotes in detail, and give most important and relevant information for eukaryotic translation. With the advances in molecular biology, the process of protein biosynthesis in eukaryotes is better understood now.
Translation in eukaryotes is briefly described here, along with some relevant features of prokaryotic protein biosynthesis. Translation proper is divided into three stages—initiation, elongation and termination (as it is done for transcription).
Initiation of Translation:
ADVERTISEMENTS:
The initiation of translation in eukaryotes is complex, involving at least ten eukaryotic initiation factors (elFs). Some of the elFs contain multiple (3-8) subunits. The process of translation initiation can be divided into four steps (Fig. 4.17).
1. Ribosomal dissociation.
2. Formation of 43S pre-initiation complex.
ADVERTISEMENTS:
3. Formation of 48S initiation complex.
4. Formation of 80S initiation complex.
Ribosomal dissociation:
The 80S ribosome dissociates to form 40S and 60S subunits. Two initiating factors namely elF-3 and elF-1A bind to the newly formed 40S subunit, and thereby block its re-association with 60S subunit. For this reason, some workers name elF-3 as anti-association factor.
Formation of 43S pre-initiation complex:
A ternary complex containing met-tRNA’ and elF-2 bound to GTP attaches to 40S ribosomal subunit to form 43S pre-initiation complex. The presence of elF-3 and elF-1 A stabilizes this complex (Note; Met-tRNA is specifically involved in binding to the initiation condon AUGs; hence the superscrip’ is used in met-tRNA’).
ADVERTISEMENTS:
Formation of 48S initiation complex:
The binding of mRNA to 43S pre-initiation complex results in the formation of 48S initiation complex through the intermediate 43S initiation complex. This, however, involves certain interactions between some of the elFs and activation of mRNA.
elF-4F complex is formed by the association of elF-4G, elF-4A with elF-4E. The so formed elF-4F (referred to as cap binding protein) binds to the cap of mRNA. Then elF-4A and elF-4B bind to mRNA and reduce its complex structure. This mRNA is then transferred to 43S complex. For the appropriate association of 43S pre-initiation complex with mRNA, energy has to be supplied by ATP.
Recognition of initiation codon:
The ribosomal initiation complex scans the mRNA for the identification of appropriate initiation codon. 5′-AUG is the initiation codon and its recognition is facilitated by a specific sequence of nucleotides surrounding it. This marker sequence for the identification of AUG is called as Kozak consensus sequences. In case of prokaryotes the recognition sequence of initiation codon is referred to as Shine- Dalgarno sequence.
Formation of 80S initiation complex:
ADVERTISEMENTS:
48S initiation complex binds to 60S ribosomal subunit to form 80S initiation complex. The binding involves the hydrolysis of GTP (bound to elF-2). This step is facilitated by the involvement of elF-5. As the 80S complex is formed, the initiation factors bound to 48S initiation complex are released, and recycled. The activation of elF-2 requires elF-2B (also called as guanine nucleotide exchange factor) and GTP. The activated elF-2 (i.e. bound to GTP) requires elF-2C to form the ternary complex.
Regulation of initiation:
The elF-4F, a complex formed by the assembly of three initiation factors controls initiation, and thus the translation process. elF-4E, a component of elF-4F is primarily responsible for the recognition of mRNA cap. And this step is the rate-limiting in translation. elF-2 which is involved in the formation of 43S pre-initiation complex also controls protein biosynthesis to some extent.
Initiation of translation in prokaryotes:
The formation of translation initiation complex in prokaryotes is less complicated compared to eukaryotes. The 30S ribosomal subunit is bound to initiation factor 3 (IF-3) and attached to ternary complex of IF-2, formyl met-tRNA and GTP.
Another initiation factor namely IF-I also participates in the formation of pre-initiation complex. The recognition of initiation codon AUG is done through Shine-Dalgarno sequence. A 50S ribosome unit is now bound with the 30S unit to produce 70S initiation complex in prokaryotes.
Elongation of Translation:
Ribosomes elongate the polypeptide chain by a sequential addition of amino acids. The amino acid sequence is determined by the order of the codons in the specific mRNA. Elongation, a cyclic process involving certain elongation factors (EFs), may be divided into three steps (Fig. 4.18).
1. Binding of aminoacyl t-RNA to A-site.
2. Peptide bond formation.
3. Translocation.
Binding of aminoacyl—tRNA to A-site:
The 80S initiation complex contains met-tRNA1 in the P-site, and the A-site is free. Another aminoacyl-tRNA is placed in the A-site. This requires proper codon recognition on the mRNA and the involvement of elongation factor 1a (EF-la) and supply of energy by GTP. As the aminoacyl-tRNA is placed in the A-site, EF-1a and GDP are recycled to bring another aminoacyl- tRNA.
Peptide bond formation:
The enzyme peptidyltransferase catalyses the formation of peptide bond (Fig. 4.19). The activity of this enzyme lies on 28S RNA of 60S ribosomal subunit. It is therefore the rRNA (and not protein) referred to as ribozyme that catalyses the peptide bond formation. As the amino acid in the aminoacyl-tRNA is already activated, no additional energy is required for peptide bond formation.
The net result of peptide bond formation is the attachment of the growing peptide chain to the tRNA in the A-site.
Translocation:
As the peptide bond formation occurs, the ribosome moves to the next codon of the mRNA (towards 3′-end). This process called translocation, basically involves the movement of growing peptide chain from A-site to P-site. Translocation requires EF-2 and GTP. GTP gets hydrolysed and supplies energy to move mRNA. EF-2 and GTP complex recycles for translocation.
In recent years, another site namely exist site (E-site) has been identified in eukaryotes. The deacylated tRNA moves into the E-site, from where it leaves the ribosome. In case of prokaryotes, the elongation factors are different, and they are EF-Tu, EF-Ts (in place of EF-1a) and EF-G (instead of EF-2).
Incorporation of amino acids:
It is estimated that about six amino acids per second are incorporated during the course of elongation of translation in eukaryotes. In case of prokaryotes, as many as 20 amino acids can be incorporated per second. Thus the process of protein/polypeptide synthesis in translation occurs with great speed and accuracy.
Termination of Translation:
Termination is a simple process when compared to initiation and elongation. After several cycles of elongation, incorporating amino acids and the formation of the specific protein/polypeptide molecule, one of the stop or termination signals (UAA, UAG and UCA) terminates the growing polypeptide.
The termination codons which act as stop signals do not have specific tRNAs to bind. As the termination codon occupies the ribosomal A-site, the release factor namely eRF recognizes the stop signal. eRF-GTP complex, in association with the enzyme peptidyltransferase, cleaves the peptide bond between the polypeptide and the tRNA occupying P-site.
In this reaction, a water molecule, instead of an amino acid is added. This hydrolysis releases the protein and tRNA from the P-site. The 80S ribosome dissociates to form 40S and 60S subunits which are recycled. The mRNA is also released.
Inhibitors of Protein Synthesis:
Translation is a complex process and it has become a favorite target for inhibition by antibiotics. Antibiotics are the substances produced by bacteria or fungi which inhibit the growth of other organisms. Majority of the antibiotics interfere with the bacterial protein synthesis and are harmless to higher organisms. This is due to the fact that the process of translation sufficiently differs between prokaryotes and eukaryotes. The action of a few important antibiotics on translation is described here.
Streptomycin:
Initiation of protein synthesis is inhibited by streptomycin. It causes misreading of mRNA and interferes with the normal pairing between codons and anticodons.
Tetracycline:
It inhibits the binding of aminoacyl tRNA to the ribosomal complex. In fact, tetracycline can also block eukaryotic protein synthesis. This, however, does not happen since eukaryotic cell membrane is not permeable to this drug.
Puromycin:
This has a structural resemblance to aminoacyl tRNA. Puromycin enters the A site and gets incorporated into the growing peptide chain and causes its release. This antibiotic prevents protein synthesis in both prokaryotes and eukaryotes.
Chloramphenicol:
It acts as a competitive inhibitor of the enzyme peptidyltransferase and thus interferes with elongation of peptide chain.
Erythromycin:
It inhibits translocation by binding with 50S subunit of bacterial ribosome.
Diphtheria toxin:
It prevents translocation in eukaryotic protein synthesis by inactivating elongation factor eEF2.
Stage # 4. Chaperones and Protein Folding:
The three dimensional conformation of proteins is important for their biological functions. Some of the proteins can spontaneously generate the correct functionally active conformation e.g. denatured pancreatic ribonuclease. However, a vast majority of proteins can attain correct conformation, only through the assistance of certain proteins referred to as chaperones.
Chaperones are heat shock proteins (originally discovered in response to heat shock). They facilitate and favour the interactions on the polypeptide surfaces to finally give the specific conformation of a protein. Chaperones can reversibly bind to hydrophobic regions of unfolded proteins and folding intermediates.
They can stabilize intermediates, prevent formation of incorrect intermediates, and also prevent undesirable interactions with other proteins. All these activities of chaperones help the protein to attain compact and biologically active conformation.
Types of Chaperones:
Chaperones are categorized into two major groups:
1. Hsp70 system:
This mainly consists of Hsp70 (70-kDa heat shock protein) and Hsp40 (40- kDa Hsp). These proteins can bind individually to the substrate (protein) and help in the correct formation of protein folding.
2. Chaperonin system:
This is a large oligomeric assembly which forms a structure into which the folded proteins are inserted. The chaperonin system mainly has Hsp60 and Hsp10 i.e. 60 kDa Hsp and 10 kDa Hsp. Chaperonins are required at a later part of the protein folding process, and often work in association with Hsp70 system.
Protein Misfolding and Diseases:
The failure of a protein to fold properly generally leads to its rapid degradation. Cystic fibrosis (CF) is a common autosomal recessive disease. Some cases of CF with mutations that result in altered protein (cystic fibrosis trans membrane conductance regulator or in short CFTR) have been reported. Mutated CFTR cannot fold properly, besides not being able to get glycosylated or transported. Therefore, CFTR gets degraded.
Certain neurological diseases which are due to cellular accumulation of aggregates of misfolded proteins or their partially degraded products have been identified. The term prions (proteinous infectious agents) is used to collectively represent them. Prions exhibit the characteristics of viral or microbial pathogens and have been implicated in many diseases, e.g. mad cow disease, Creutzfeldt- Jacob disease, Alzheimer’s disease, Huntington’s disease.
Stage # 5. Post-Translational Modifications of Proteins:
The proteins synthesized in translation are, as such, not functional. Many changes take place in the polypeptides after the initiation of their synthesis or, most frequently, after the protein synthesis is completed. These modifications include protein folding, trimming by proteolytic degradation, intein splicing and covalent changes which are collectively known as post-translational modifications (Fig. 4.20).
Proteolytic Degradation:
Many proteins are synthesized as the precursors which are much bigger in size than the functional proteins. Some portions of precursor molecules are removed by proteolysis to liberate active proteins. This process—commonly referred to as trimming- may occur in Golgi apparatus, secretory vesicles and, sometimes, after the secretion of proteins.
The formation of insulin from pre-pro-insulin, conversion of zymogens (inactive digestive enzymes e.g. trypsinogen) to the active enzymes are some examples of trimming. The synthesis of the proteins as inactive precursors and their later conversion into active form, may be, to protect the functional protein unit from the environmental insults.
Intein Splicing:
Inteins are intervening sequences in certain proteins. These are comparable to introns in mRNAs. Inteins have to be removed, and exteins ligated in the appropriate order for the protein to become active.
Covalent Modifications:
The proteins synthesized in translation are subjected to many covalent changes. By these modifications in the amino acids, the proteins may be converted to active form or inactive form.
Selected examples of covalent modifications are described below:
1. Phosphorylation:
The hydroxyl group containing amino acids of proteins, namely serine, threonine and tyrosine are subjected to phosphorylation. The phosphorylation may either increase or decrease the activity of the proteins. A group, of enzymes called protein kinases catalyses phosphorylation while protein phosphatases are responsible for dephosphorylation (removal of phosphate group). Many examples of enzymes that undergo phosphorylation or dephosphorylation are known in metabolisms.
2. Hydroxylation:
During the formation of collagen, the amino acids pro-line and lysine are respectively converted to hydroxyproline and hydroxylysine. This hydroxylation occurs in the endoplasmic reticulum and requires vitamin C.
3. Glycosylation:
The attachment of carbohydrate moiety is essential for some proteins to perform their functions. The complex carbohydrate moiety is attached to the amino acids, serine and threonine (O-linked) or to asparagine (N-linked), leading to the synthesis of glycoproteins. Vitamin K dependent carboxylation of glutamic acid residues in certain clotting factors is also a post-translational modification.
In the Table 4.2, selected examples of post- translational modification of proteins through their amino acids are given.