ADVERTISEMENTS:
This article throws light upon the thirteen types of tools and techniques used for plant tissue culture.
The applications and principle of each of the tools and techniques used for plant tissue culture are briefly described hereunder with schematic diagrams.
1. pH and pH Meter:
The hydrogen ion concentration of most solutions is extremely low. In 1909, Sorenson introduced the term pH as a convenient way of expressing hydrogen ion concentration. pH of a solution is strictly defined as the negative logarithm of the hydrogen ion activity. But in practice usually hydrogen ion concentration is taken.
ADVERTISEMENTS:
pH = – log10 (H+) = 7
The pH of pure water is 7 at 25 °C. Generally glass distilled water is used for the preparation of culture medium. However, sometimes buffered solutions may be used for the same to keep the pH of the medium constant.
Measurement of pH:
An approximate idea of the pH of a solution can be obtained using indicators. These are organic compounds of natural or synthetic origin whose Colour is dependent upon the pH of the solution. Indicators are usually weak acids, which dissociate in solution. A standard pH meter has two electrodes, one glass electrode for measuring pH and the other calomel reference electrode (Fig. 25.1). Reference electrode is filled with saturated KCI solution.
Indicator = Indicator– + H+
ADVERTISEMENTS:
The pH probe measures pH as the activity of hydrogen ions surrounding a thin walled glass bulb at its tip. The probe produces a small voltage (about 0.06 volt per pH unit) that is measured and displayed as pH units by the meter. The meter circuit is fundamentally no more than a voltmeter that displays measurements in pH units instead of volts.
The input impedance of the meter must be very high because of the high resistance approximately 20 to 1000 MΩ of the glass electrode probes typically used with pH meters. The circuit of a simple pH meter usually consists of operational amplifiers in an inverting configuration, with a total voltage gain of about – 17. The inverting amplifier converts the small voltage produced by the probe (+ 0.059 volt/pH) into pH units, which are then offset by seven volts to give a reading on the pH scale.
For example:
(i) At neutral pH (pH 7) the voltage at the probe’s output is 0 volts.
(ii) At alkaline pH, the voltage at the probe’s output ranges from + 0 to + 0.41
(iii) At acid pH, the voltage at the probe’s output ranges from – 0.41 volts to – 0.
(iv) Now-a-days in more sophisticated pH meters, the both types of the electrodes are combined in one.
Applications:
1. To adjust pH of different solutions, preparation of buffers and culture media.
2. Determination of pH of cells (cell sap) and in analytical techniques.
ADVERTISEMENTS:
3. To monitor pH of the medium in a bioreactor.
2. Autoclave:
Autoclave is used to sterilize medium, glassware and tools for the purpose of plant tissue culture. The same equipment is used in hospitals to sterilize gauge, cotton, tools and linen, etc. Sterilization of material is carried out by increasing moist heat (121 °C) due to increased pressure inside the vessel (15-22 psi, pounds per square inch or 1.02 to 1.5 kg/cm2) for 15 minutes for routine sterilization. Moist heat kills the microorganism and makes the material free from microbes.
Construction:
Autoclaves of different sizes from 5 litres to several hundred litres capacity are available in horizontal or vertical designs. As shown in Fig. 25.2, an autoclave have a body, an internal (or external for small sized autoclaves comparable to household pressure cooker) heating system, a container to hold material, its cover fixed with pressure gauge, safety valve, pressure release valve etc. Lid is tightened with the help of screws and a gasket seals the body and lid. A jacket, paddle lifter, timer, and indicator etc., are also provided with large sized autoclaves. Autoclaves may be constructed of aluminum, mild steel, stainless steel or gun metal. Industrial autoclave can accommodate large trolley containing huge number of glassware’s or large bioreactors.
 Operation:
Operation:
Place the materials (wrapped in aluminum foil or paper or in metal box) and glass-wares containing medium (plugged with non-absorbent cotton and covered with aluminum foil) in the bucket. Check water level for appropriate level, tighten all the screws, and switch-on the current. Allow the steam to pass freely from release valve for 5 minutes and then close the valve.
ADVERTISEMENTS:
After attaining a pressure of 15 psi, count 15 minutes for sterilization and then switch-off the current. Pressure is maintained by safety valve. Modern autoclaves are fitted with temperature and time control units and can automatically control the period for sterilization and then switch-off themselves.
Empty vessels, beakers, graduated cylinders, etc., should be closed with a cap or aluminum foil. Tools should also be wrapped in foil or paper or put in a covered sterilization tray. It is critical that the steam penetrate the items in order for sterilization to be successful.
For large sized vessels and large volume flasks containing high amount of liquid, duration for sterilization should be increased accordingly. Table 25.1 shows the relationship between volume of the solution and duration for its sterilization at 15 psi. The vessels should not be filled more than 1/3 of its capacity for proper sterilization.
Precautions:
1. Check water level each time, the heating elements should remain immersed in the water.
2. Check spring of safety valve frequently and clean opening whenever necessary.
3. Opposite screws of the lid should be tightened simultaneously.
4. Do not over tighten the screws to avoid damage to the gasket.
ADVERTISEMENTS:
5. Use permanent marker to mark your flasks and its medium.
6. All the electrical equipment should be properly earthen to avoid electric shock.
3. Plant Growth Chamber:
Plant growth chambers can be constructed in a suitable sized room or can be purchased as commercially available equipment. Thermal insulation of walls increases the efficiency of the cooling system.
Essentially plant growth chamber has three environmental control systems:
1. Light-intensity and duration cycle control.
2. Temperature control and regulation.
ADVERTISEMENTS:
3. Humidity control and regulation.
All the modern instruments are electronically controlled precision instruments with sophisticated sensors and timers to regulate the desired set values.
(1) Light:
Light is fixed in the roof of equipment or in shelves. Light is provided by commercially available light sources like cool white fluorescent tubes and incandescent lamps in a ratio of 3:1 and usually a light intensity of 2000-2500 lux (about 200-250 candles or 30 µ mol m-2 s1) is used. The duration of light and dark cycle is adjusted as per requirement, usually 16 hours light cycle is given. Nowadays warm fluorescent tubes are also available which provides wide spectrum as compared to cool white fluorescent tubes and mixing of incandescent light is not required with former tubes. Light intensity can also be regulated by photoperiod simulators. Thus, light quality, intensity and period is controlled and regulated by the instrument as per set valves.
(2) Temperature:
In modern equipment, temperature is precisely regulated by good quality (platinum) temperature sensor. In all cases, air conditioning units provide the cooling. It is always advisable to keep one spare compressor unit, for emergency, to avoid delay in repairs and damage to cultures. Usually temperature of 22-28 °C is used for growing plant tissue culture. Temperatures should be measured in a constructed growth chamber at different levels and places, viz., light racks, central and corners to have a correct temperature setting.
(3) Humidity:
Humidity inside the growth chamber is provided by humidifier (a mist generating system) and controlled by humidistat. Usually 60% RH (relative humidity) is used to maintain healthy growth. Low RH may cause early drying of medium while high humidity may cause fungal growth in the environment and on a various articles. Thus, in a growth chamber, light, temperature and humidity are precisely controlled and cultures are grown in a controlled environment. All the controls are set on control panel.
4. Laminar Air Flow Bench:
Laminar air flow (LAF) bench is the main working table for aseptic manipulations related to plant tissue culture. This is equipment fitted with High Efficiency Particulate Air (HEPA) Filters, which allow air to pass but retain all the particles and micro-organisms. These HEPA Filters have a very small pore size (0.3 µm) with 99.97-99.99% efficiency.
ADVERTISEMENTS:
Air blown by a blower is passed through these filters, thus, always generally a positive pressure of air is maintained from inside to outside. This positive pressure does not allow any particle to enter in the working area of LAF bench. Air pressure inside the instrument is measured by a manometer and pressure of more than 12 bars shows choking of filters and at this point filters should be replaced. Equipment is fitted with UV light and visible light source.
UV is switched-on for 30 minutes before starting the work to make area free from microbes. LAF Bench of steel and wooden cabinets is available with different working table size and for vertical (downward air flow) or horizontal (horizontal flow) model. There are several manufacturers in India for this instrument (body) but HEPA filters are imported (Fig. 25.3). HEPA filters are also used to create ‘clean area’ for culture rooms and inoculation room etc. If LAF bench is placed in such a clear area, efficiency and life of the equipment are increased.
5. Microscopy:
(a) Electron Microscopy:
Electron microscopy permits a detailed study of sub-cellular organelles as its resolving power is much greater than that of the light microscope. Max Knoll and Ernst Ruska in 1931, at Technical University in Berlin, constructed electron microscope (EM). In the EM, streams of electrons are deflected by an electrostatic or electromagnetic field in the same way that a beam of light is refracted by a lens.
Electron beam is generated by heating a filament in vacuum, which are accelerated by a potential and shows properties similar to light (λ = 0.005nm of electrons and 550 nm for light). Though appears to be similar there are great differences in light and electron microscopes, the principal being the image formation (Fig. 25.4.). In electron microscope, image is produced by electron scattering.
Electron dispersion is a function of the thickness and molecular packing of the object and depends especially on the atomic number of the atoms in the object. The higher the atomic number, the greater is the dispersion. There are three general types of electromagnetic lenses.
The one is placed between the source of illumination and the specimen. This focuses the beam of electrons on specimen and functions in a similar manner as the condenser in light microscope. The other two lens systems are on the opposite side of specimen which magnifies the image in similar way as objective and ocular in light microscope.
The final image is produced on a screen coated with a phosphorus compound which fluoresces upon irradiation by electrons. These images are recorded on photographic films. Air molecules in the microscope interfere with the movement of electrons. To prevent this, a high vacuum (10-4 – 10-6 mm Hg) is created inside the microscope.
In light microscope, magnification is largely determined by the objective and the maximum magnification of 100-120 can be obtained. This is multiplied by ocular lens by a factor of 5-15, reaching a total magnification of 500-1500x.
The resolving power of Transmission Electron Microscope (TEM) is very high. The image generated by objective can be multiplied several hundred times by projector coil, e.g., 100 objective 200x projector coil = 20,000x. In TEM, this can be reached up to 10,000,000 xs. Electron microscope has a greater depth of field as compared to light microscope. This property of EM is used to develop three dimensional images of cell organelles as ribosome’s and protein structure.
The preparation of specimen is similar to dehydration used for histology for light microscopy. The sections prepared for EM should be thin (less than 0.5 or 500 nm), otherwise, material will appear completely dark. Such thin sections (50-300 nm) are prepared with the help of ultra-microtome, fitted with glass or diamond knife. To cut sections, material is fixed in osmium tetra oxide, processed through a dehydration series and embedded in plastic or resin (araldite).
Sections are placed on small grids specifically used for EM. It is difficult to handle ultra thin sections so they are placed on copper grids (400 apertures/inch square).The grids are placed in labeled boxed and can be stored till observed in EM and can be used again and again without damaging the material. Dehydrated materials are placed in vacuum chamber and vapour of heavy metals like platinum, chromium or palladium is directed from a filament of incandescent tungsten.
The vaporized material is deposited on one side of the surface of elevated particles. This creates a shadow on the other side (Shadow casting). Height of the material is determined by length of shadow. In photographs, such specimens appear in three dimensions, which is not possible with other techniques.
Freeze fracturing (fracture of frozen material) is used to see fine details of replicated material. These are different techniques to prepare materials for sectioning and for staining to increase contrast. Special techniques are used for bacteria, viruses, proteins and nucleic acids. TEM is used to study the fine details of a cell, compare the clones, development of plastids and cell wall formation (Figs. 25.5, 25.6).
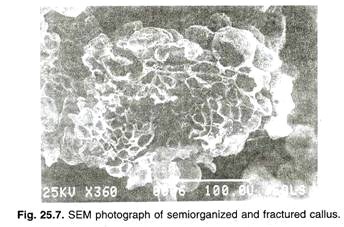
(b) SEM:
Scanning electron microscope (SEM) provides surface views of whole structure of specimen (Fig. 25.7). Normally, specimens are coated with a thin film of metal under vacuum. As compared to shadow casting, complete coating is essential because the scanning beam produces a charge on the uncoated biological materials which cause distortion of image.
The metal coated specimen is scanned with a beam of electrons (50-100 Å) which strikes on the specimen and emits the secondary electrons. These electrons pass through a cathode tube and image is produced on the screen of Cathode Ray Tube. In plant system, SEM is used to see the nature of appendages, pollen morphology and morphogenesis in plant tissue culture. It is also used to study the structures of metals, crystals and in forensic sciences.
(c) Light Microscopy:
Bright field microscopy is absolutely indispensable tool for cell biologists. This is required for routine observations of cells, cellular differentiation and pigmentation. Fluorescence microscopy has become a powerful tool for cell biologists, particularly for the selection of fluorescing secondary metabolite rich cells. Only these two techniques are discussed here in brief.
According to wave theory, light is propagated from one place to another as wave travelling in a hypothetical medium. Light waves are described in terms of their amplitude, frequency, and wavelength. Amplitude is the maximum displacement of light path from the position of equilibrium.
Frequency of light is the number of complete cycles occurring in a second. The property of light waves inversely associated with the frequency is the wavelength and is defined as the distance between corresponding points on a wave or distance between two successive peaks or crests. The velocity of light is about 186300 miles or 3 x 1015 kms per second.
A good microscope has not only good magnifying power but also good resolving power to provide finer details of the object. Thus, the basic difficulty in designing a microscope is riot the magnification, but the ability of lens system to distinguish two adjacent points as distinct and separate. This ability is known as resolving power of the microscope. The resolving power of a microscope depends upon the wavelength of light and numerical aperture (N.A.).
The minimum resolvable distance between two luminous points (v) is given by the following formula:
Thus, shorter the wavelength of light used and lower the N.A., greater is the resolving power. The limit of resolution of a microscope is approximately equal to 0.5 /N.A., which for a light microscope is approximately 200 nanometers (nm) or about the size of many bacterial cells.
The numerical aperture of a lens is dependent on the refractive index (r) = the ratio of the speed of light in a given medium to the speed of light in a vacuum) of the medium filling the space between the specimen and the front of the objective lens and on the angle of the most oblique rays of light that can enter the objective lens (θ). It is given by formula [N.A. = ηx sinθ], that is why immersion oil is placed between the object and oil immersion lens (100 X objective).
6. Colorimeter:
The most commonly used method for determining the concentration of biochemical compounds is colorimetry. It uses the property of light such that when white light passes through a coloured solution, some wavelengths are absorbed more than others. Hyaline solution can be made coloured by specific reactions with suitable reagents. These reactions are generally very sensitive to determine quantities of material in the region of millimole per litre concentration.
The big advantage is that complete isolation of the compound is not necessary and the constituents of a complex mixture such as blood can be determined after little treatment. The depth of colour is directly proportional to the concentration of the compound being measured, while the amount of light absorbed is proportional to the intensity of the colour and therefore, to the concentration.
Source of Radiation:
A lamp is usually used as the source of radiation. The wavelength of light depends upon the quality of source lamp. Colorimetry is an analytical device to determine the amount of an unknown substance in a solution. The device works on optical properties of the substance. Basically we measure the amount of light of a particular wavelength absorbed by that solution, and then relate solute concentration to the absorbance.
A simplified schematic presentation of the instrument is as follows:
The relationship between concentration of solution and light it absorbs is given by the following laws.
Lambert’s Law:
When a ray of monochromatic light passes through an absorbing medium its intensity decreases exponentially as the length of the absorbing medium increases.
Beer’s Law:
When a ray of monochromatic light passes through an absorbing medium its intensity decreases exponentially as the concentration of the absorbing medium increases.
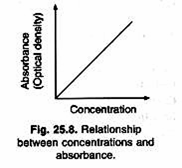
Transmittance:
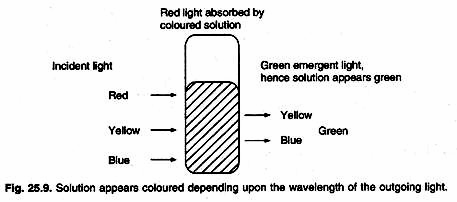
The ratio of intensities is known as the transmittance and this is usually expressed as percentage. Absorbance or extinction is known as the optical density of the substance. Absorbance increases with increase in concentration of the solution (Fig. 25.8).
Detection of Radiation:
Detection wavelength is selected on the basis of wavelength of light coming out of a solution (Fig. 25.9). Absorption of a particular wavelength of light is characteristic of a compound. Outgoing radiations are detected by photochemical detectors or vacuum phototubes. If the light intensity is very low, a photomultiplier tube is used. A phototube converts the transmitted light energy into an electric current. This electric signal is calibrated and can be produced on a chart recorder.
A better method is to split the light beam, pass one part through the sample and the other through the blank, and balance the two circuits to give zero (a double beam system). The extinction is determined from the potentiometer reading, which balances the circuit.
Applications:
Measurement of concentrations:
Spectrophotometers are widely used for quantitative measurements of substance concentration in the solutions by measuring absorbance at the optimal wavelength. This is used to determine concentration in fractions obtained in column chromatography, TLC and in other solutions.
Absorption spectra:
Identification and structure evaluation of various isolated pure compounds can be done by UV-Visible spectrophotometry. All the compounds have very specific absorption spectra depending on the molecular structure. Comparison of spectra helps in identification of compounds. The wavelength at which maximum absorption takes place is represented as λmax of that compound in a given solvent.
Enzyme kinetics:
In enzymatic reactions, changes in absorbance are recorded to determine the reaction velocity and concentration of the product formed/substrate utilized. Spectrophotometers coupled with microprocessor or computer can store data, figures and are helpful in comparing the effect of different variables.
7. Centrifugation:
A centrifuge is an instrument which produces centrifugal force by rotating the samples around a central axis with the help of an electric motor. Centrifuges can be categorized as the clinical type (5-10,000 rpm), refrigerated high-speed centrifuges (10,000-20,000 rpm) and ultra -centrifuges (20,000 to 80,000 rpm).
With increase in rpm, the friction of rotor with air produces so much of heat that they have to be run under refrigeration (so called refrigerated centrifuge) and both refrigeration and vacuum are used in ultracentrifuge, which runs at very high rpm. For these high speeds, even the rotor has to be made of special metal to withstand the great force.
There are two types of rotors, angle head and swing (Fig. 25.10). In the former, the samples are kept at an angle of about 30° to the vertical axis whereas; in the latter the samples while spinning are horizontal. Simple calculations show that for the same radius, the swinging bucket method produces more gravitational force. Ultracentrifuges are of two types – analytical and preparative model.
Analytical Model:
This consists of rotors and tubes, called cells. The instrument is designed to allow the operator to follow the progress of the substances in the cells, while the process of centrifugation is in progress. By estimating sedimentation velocity during the process, the molecular weight, purity etc. can be determined.
Preparative Model:
This is used for purification of the components of macromolecules or other substances and all determinations are made at the end of centrifugation. The instrument has no monitoring device, while large centrifugal forces are set for a fixed time period. Centrifugation is the most widely used technique for separation of various metabolites and also used to separate non-miscible liquids during extraction of secondary metabolites, e.g., water (aqueous), chloroform (organic) mixture.
A wide variety of centrifuges are available, ranging in capacity and speed. During the process of centrifugation, solid particles experience a centrifugal force, which pulls them outwards, i.e., away from the center. The velocity with which a given solid particle moves through a liquid medium is related to angular velocity. The principle of centrifugation is that, an object moving in a circular motion at an angular velocity is subjected to an outward force (F) through a radius of rotation (r) in cms, presented as ω2[F = ω2r].
F frequently expressed in terms of gravitational force of the Earth, commonly referred to as RCF (Relative centrifugal force) or g by the following formula [RCF = ω2r / 980]. The ω2 operating speed of the centrifuge is expressed as revolutions per minute ‘rpm’, which can be converted to radians by the following formula:
Sometimes the velocity of the moving particles is expressed in the form of sedimentation coefficient (s) by the formula [V – s (ω2r)]. The sedimentation coefficient is a characteristic constant for a molecule or a particle and is a function of the size, shape and density. It is equivalent to the average velocity per unit of acceleration. The unit Svedberg (s) is often used with reference to centrifugation and is equivalent to a sedimentation coefficient of 10-13 s.
Applications:
Centrifugation is widely used in analytical techniques, preparation of extracts, separation of non-miscible liquid mixtures, purification of enzymes, inhibitors and removing particles (silica, etc.) from the solvents. Centrifugation with heating and vacuum (suction) is used for concentrating extraction (instrument called sample concentrator) and rapidly removing solvents. All the enzymatic work requires refrigerated centrifuges to keep the samples cool during centrifugation and to protect enzymes from inactivation by heat.
8. Chromatography:
Chromatography (meaning ‘colored writing’) is a technique to separate molecules on the basis of differences in size, shape, mass, charge and adsorption properties. The term chromatography was used by the Russian botanist Tswett to describe the separation of plant pigments on a column of alumina. There are different types of chromatography but they all involve interactions between these components: the mixture to be separated, a solid phase, and a solvent.
The magnitude of these interactions depends upon the particular method used. Usually column chromatography is used to separate large quantities of compounds, whereas, paper chromatography (PC) or thin-layer chromatography (TLC) in one or two dimensions, is used for analytical work.
The mobile phase can be a gas or a liquid, whereas the stationary phase can only be a liquid or solid. In liquid column chromatography (LCC), separation involves a simple partitioning between two immiscible liquid phases, one stationary and the other mobile, the process is called liquid-liquid (or partition) chromatography (LLC). When physical surface forces are mainly involved in the retentive ability of the stationary phase, the process is denoted liquid-solid (or adsorption) chromatography (LSC).
In ion exchange chromatography (IEC), ionic components of the sample are separated by selective exchange with counter ions of the stationary phase. Use of exclusion packing’s as the stationary phase brings about a classification of molecules based largely on molecular geometry and size.
Paper Chromatography:
Principle:
Cellulose in the form of paper sheets makes an ideal support medium where water is absorbed between the cellulose fibres and forms a stationary hydrophilic phase. The suitably concentrated mixture is spotted onto the paper, dried with a hair-drier and the chromatogram is developed by allowing the solvent to flow along the sheet. The solvent front is marked and after drying the paper, the positions of the compounds present in the mixture are visualized by a suitable staining reaction.
The ratio of the distance moved by a compound to that moved by the solvent is known as the R; value and is more or less constant for a particular compound, solvent system and paper under carefully controlled conditions of solute concentration, temperature and pH.
Sample:
Generally, alcoholic extracts of plant material with or without partial purification are used for chromatography. Biological materials should be desalted before chromatography by electrolysis or electro-dialysis. Excess salt results in a poor chromatogram with spreading of spots and changes in their R. values.
It can also affect the chemical reactions used to detect the compounds being separated. The sample (10-20 µl) is then applied to the paper with a micropipette or capillary.
Paper:
Whatman No. 2 is the paper most frequently used for analytical purposes. Whatman No. 3 MM is a thick paper and is best employed for separating large quantities of material; the resolution is, however, inferior to Whatman No. 1. For rapid separation, Whatman Nos. 4 and 5 are convenient, although the spots are less well defined. In all cases, the flow rate is faster in the ‘machine direction’, which is normally noted on the box containing the paper.
The paper may be impregnated with a buffer solution before use or chemically modified by acetylation. Ion exchange papers are also available commercially. For the separation of lipids and similar hydrophobic molecules, silica-impregnated papers are available commercially.
Solvent:
This choice, like that of the paper, is largely empirical and will depend on the mixture investigated. If the compounds move close to the solvent front in solvent ‘A’ then they are too soluble, while if they are crowded around the origin in solvent ‘B’ then they are not sufficiently soluble.
A suitable solvent for separation would, therefore, be an appropriate mixture of ‘A’ and ‘B’, so that the R ƒ values of the components of the mixture are spread across the length of the paper.
R ƒ value is defined as [R ƒ = the distance moved by solute / the distance moved by solvent front]. This value is a constant for a particular compound under standard conditions and closely reflects the distribution coefficient for that compound. It is recommended that the developing chamber should be saturated with solvent, by using filter paper lining inside the chamber.
Two Dimensional Chromatography:
The mixture is separated in the first solvent, which should be volatile, then after drying, the paper is turned through 90° and separation is carried out in the second solvent. After location, a map is obtained and compounds can be identified by comparing their position with a map of known compounds developed under the same conditions (Fig. 25.11).
Detection of spots:
Most compounds are colourless and are visualized by specific reagents. The location reagent is applied by spraying the paper under a fumigation hood or rapidly dipping it in a solution of the reagent in a volatile solvent. Viewing under ultraviolet light is also useful since some compounds, which absorb strongly show up as dark spots against the fluorescent background of the paper. Other compounds show a characteristic fluorescence under ultraviolet light.
Thin Layer Chromatography:
Principle:
Separation of compounds on a thin layer of adsorbing material is similar in many ways to paper chromatography, but has the added advantage that a variety of supporting media can be used so that separation can be by adsorption ion exchange, partition chromatography, or gel filtration depending on the nature of the medium employed.
The method is very rapid compared to paper chromatography and many separations can be completed within an hour. Compounds can be detected at a lower concentration than on paper as the spots are very compact. Furthermore, separated compounds can be detected by corrosive sprays and elevated temperatures with some thin layer materials, which of course is not possible with paper.
Production of thin layer:
The R ƒ value is affected by the thickness of the layer below 200 µm and a thickness of 250 µm is suitable for most separations. There are several good spreaders available in the market, which can produce an even layer of required thickness by adjusting the thickness control screw.
Calcium sulphate is sometimes incorporated into the adsorbent to bind the layer to the plate and, because of this; it is advisable to work rapidly once the adsorbent is mixed with water.
There are now a number of prepared thin layer plates using different adsorbents on various supporting materials such as glass, plastic, and aluminum that are available commercially and these may be more convenient to use than trying to prepare plates in the laboratory.
Development:
It is essential to make sure that the atmosphere of the separation chamber is fully saturated with the solvent mixture, otherwise R ƒ values will vary widely from tank to tank. However, horizontal chambers of high performance TLC (HPTLC) are very useful and convenient as they require less time, solvent and no stabilization time. Development of the plate is usually by the ascending technique and is very rapid.
9. Thermometer:
The thermometer is a device that measures temperature or temperature gradient using a variety of different principles; it comes from the Greek roots thermo, heat, and meter, to measure. A thermometer has two important elements: the temperature sensor (e.g., the bulb on a mercury thermometer) in which some physical change occurs with temperature, plus some means of converting this physical change into a value (e.g., the scale on a mercury thermometer).
Industrial thermometers commonly use electronic means to provide a digital display or input to a computer. The Alcohol thermometer or Spirit thermometer is an alternative to the Mercury-in-glass thermometer, and functions in a similar way. An organic liquid is contained in a glass bulb which is connected to a capillary of the same glass and the end is sealed with an expansion bulb. The space above the liquid is a mixture of nitrogen and the vapour of the liquid.
For the working temperature range, the meniscus or interface between the liquid is within the capillary. With increasing temperature, the volume of liquid expands and the meniscus moves up the capillary. The position of the meniscus shows the temperature against an inscribed scale.
The liquid used can be pure ethanol or toluene or kerosene or Isoamyl acetate, depending on manufacturer and working temperature range. Since these are transparent, the liquid is made more visible by the addition of a red or blue dye. One half of the glass containing the capillary is usually enameled white or yellow to give a background for reading the scale.
Temperature is measured by maximum-minimum thermometer or a continuous rotary dram chart type thermometer. The U- shaped maximum-minimum thermometer is commonly used for determining diurnal maximum and minimum range of temperature in the culture room.
During the dark period, temperature remains slightly (1-2 °C) lower than light period (all bulbs and tube lights of the culture room increase the temperature). Therefore, chokes of the tube lights are fitted outside the culture room.
The indicators of the maximum-minimum thermometer are moved by mercury column and they remain at that position until moved by the observer with the help of magnet. Their positions indicate the minimum and maximum temperature in the previous 24 h. After recording temperature indicators are reset to mercury level (Fig. 25.12).
10. Hygrometer:
Hygrometers are instruments used for measuring humidity. A simple form of a hygrometer is specifically known as a “psychrometer” and consists of two thermometers, one of which includes a dry bulb and the other of which includes a bulb that is kept wet to measure wet-bulb temperature. Evaporation from the wet bulb lowers the temperature, so that the wet-bulb thermometer usually shows a lower temperature than that of the dry-bulb thermometer, which measures dry-bulb temperature.
Relative humidity is computed from the ambient temperature as shown by the dry- bulb thermometer and the difference in temperatures as shown by the wet-bulb and dry-bulb thermometers. Relative humidity can also be determined by locating the intersection of the wet- and dry-bulb temperatures on a psychrometric chart.
Dial type hair hygrometer is a convenient tool to measure humidity in the culture room. Humidifier is used to maintain 60% relative humidity (RH) in the culture room. Humidistat is a bimetallic thermocouple device to control and regulate the function of humidifier to maintain the humidity. Distilled water should be filled in the humidifier.
The RH present in the culture room is measured by hair hygrometer. As the name suggests a chemically treated hair elongates with increased humidity and shortens with dryness (similar to mercury in thermometer). It is calibrated from 0 to 100% RH. At lower humidity, medium dries rapidly whereas at higher humidity chances of fungal growth over all surfaces and cotton plugs is increased. Therefore, about 60% RH is maintained in the culture room (Fig. 25. 12).
11. LUX Meter:
Light is the form of radiant energy, i.e., electromagnetic radiation of specific wavelength. Visible light as we perceive, is located in narrow wavelength region of spectrum between 380 to 760 nm. Light has dual characters, displaying both wave properties (refraction, diffraction, interference and polarization phenomena) and particle properties (light is radiated in discrete amounts of energy or photons).
Properties of light should be defined either as irradiance (radiant flux intercepted per unit area; unit = w/m2) or an illuminance (luminous flux intercepted per unit area, unit lux, 10.76 lux = 1 foot candle). It is to note that an irradiance measurement is not spectrally defined, whereas, an illuminance measurement indicates the level of visible light as the human eye would see it.
Artificial light is provided by cool, white, fluorescent tubes and/or incandescent bulbs of different classes of artificial light sources, fluorescent sources have been used almost exclusively for plant tissue culture because they are more efficient at producing broad band visible light than incandescent bulbs and are available in low output wattage than lamps.
Light intensity can be measured by photometer or lux meter. A photometer consists of photoelectric cell and a micro-ammeter. Photoelectric cell is sensitive for light and converts light into current. Micro-ammeter shows reading due to this current and its needle moves. Nowadays digital read out is given by appropriately converting the current into digital signal (Fig. 25.12). High intensity is proportional to the current generated in the photoelectric cell by falling light. The unit of illumination is called as ‘lux’ and the scale of ammeter is calibrated in lux.
Usually 2000 to 2500 lux is provided to the cultures maintained in light in the culture room. The instrument is a sensitive tool and handled with care. It should not be exposed to the sunlight without switching the proper reading switch to high light illumination.
12. Methods of Sterilization:
Asepsis:
Plant tissue culture requires contamination free environment, tools and cultures or strict maintenance of germ free system in all the operations, known as asepsis. Particularly in
commercial production units, the contamination of one batch of the cultures may result in heavy financial losses or even loss of a culture strain. Therefore strict control measures are enforced to maintain the entry of the personnel and living materials. The basic rules and practices of asepsis are followed in all the tissue culture laboratories.
Plant tissue culture media are rich in nutrients and very suitable for the growth of microbes also. These microorganisms grow faster, consume nutrients rapidly and suppress the growth of plant tissues (by over growth). This saprophytic unwanted growth of microbes is formed as contamination. There are many ways by which these microbes suppress the growth of plant cells and tissues, e.g., release of enzymes and toxins (which inhibit the growth of plant cells), selective absorption of specific nutrients, and some tissue infection.
To achieve success in cultivation of higher plant parts, it is essential to exclude these contaminating microorganisms and hence aseptic techniques must be employed to save cultures. This has led to the development of ‘clean area concept’. All the area, tools and working places should be free from microbes to have less and less problems of contamination.
Following care should be taken:
1. Minimize the air current in the working area so it is possible to avoid spores of contaminating microorganisms to move in along with the air currents over the sterile areas. At least, fan should not be used in laminar air flow bench room (inoculation room). Preferably, all the places should be air-conditioned.
2. Store properly the prepared media, nutrients and tools in cabinets.
3. Use separate area for cleaning and washing and for the preparation of medium.
UV tubes are also fixed in Laminar air flow bench placed in inoculation chamber. All these UV tube lights should be used frequently before inoculations. UV lights of corridors may be left open during nights.
Surface Sterilization of Explants:
Most contamination is introduced with the explant because of inadequate sterilization or just very dirty material. It can be fungal or bacterial. This kind of contamination can be a very difficult problem when the plant explants material is harvested from the field or greenhouse.
Initial contamination is obvious within a few days after cultures are initiated. Bacteria produce “ooze” on solid medium and turbidity in liquid cultures. Fungi look “furry” on solid medium and often accumulate in little balls in liquid medium.
Bacteria are the most frequent contaminants. They are usually introduced with the explants and may survive surface sterilization of the explants because they are in interior tissues. So, bacterial contamination can first become apparent long after a culture has been initiated. All explants have to be sterilized before transferring them on to the medium.
A suitable sized explants can be sterilized by any one of the following solutions:
1. 1% solution of sodium hypochlorite (commercial bleach).
2. 7 % saturated solution of calcium hypochlorite.
3. 1 % solution of bromine water.
4. 70% ethyl alcohol.
5. 0.2% mercuric chloride.
6. 10% hydrogen peroxide solution.
7. 1% silver nitrate solution.
Suitable sized plant material is sterilized as follows:
1. Clean the working area with ethanol, and start the air flow of the laminar air flow bench.
2. Sterilized petridishes, distilled water, scalpel, filter paper sheets (suitable sized and autoclaved) alcohol, disinfectant (mercuric chloride 0.01-0.1% aqueous solution, or 20% sodium hydrochloride), forceps, bead sterilizer or beaker or coupling jar, sprit lamp or gas burner are collected on Laminar flow bench. Put on the UV light of the bench and put it off after 30 minutes.
3. Bring the plant material to be sterilized on the bench and prepare pieces for sterilization.
4. Clean the working area and hands with alcohol, put on mask and cap, and light the sprit lamp.
5. Keep 3-4 petridishes in a line; add disinfectant in 1st plate and autoclaved distilled water in subsequent plates.
6. Place plant pieces in 1st plate and immerse the material with the help of forceps for 5-10 minutes depending upon the disinfectant used.
7. Transfer material from 1st to 2nd plate, rinse gently and pass to 3rd and 4th plate, one by one with thorough rinsing.
8. Finally, drain the distilled water or place the material in a fresh petridish or filter paper and prepare suitable sized explants (Fig. 25.13).
Mode of Action of Disinfectants:
The various metallic ions can be arranged in a series of decreasing antibacterial activity. Hg2+ and Ag+, are effective at less than one part per million (ppm), because of their high affinity for sulphahydryl group. Bacteria are killed by Ag containing 105 to 107 Ag+ ions per cell. The concentration required for killing is markedly affected by inoculum size.
Chlorine was the anticeptic introduced (as chlorinated lime) by O.W. Holmes in Boston in 1835 and by Semmelweis in Vienna in 1847, to prevent transmission of puerperal sepsis by the physicians hand. Chlorine combines with water to form hypochlorous acid (HOC1) a strong oxidizing agent.
CI2 + H2O = HCl + HOCl
Cl2 + 2NaOH = NaCl + NaOCl + H2O
Sodium hypochlorite (NaOCl) solutions are (200 ppm chlorine) are used to sanitize clean surfaces in the food and the dairy industries and in restaurants. Sodium hypochlorite is commercial bleach available in the market as 3-6% solution and can be used directly. It is prepared by passing chlorine in to dilute solution of sodium hydroxide. Powdered bleach is also added into water supply for killing microbes.
Equipment and Medium Sterilization:
All the manipulations of plant tissue culture methods are carried out in aseptic conditions. All the materials used are therefore, free from microbes or sterilized. Various methods of sterilization are used depending upon the material and type of sterilization.
Essentially, method for sterilization is different for living materials than tools and glass ware. Now a day’s many items are available as ready to use, pre-sterilized (by gamma radiation) and disposable. Uses of such disposable materials have facilitated the work.
In laboratory, in principle, three types of sterilization is used:
1. Dry heat
2. Wet heat
3. Filter sterilization.
1. Dry heat:
Glassware, metal tools and other articles, which do not get charred by high temperature, are put in containers or wrapped in paper or thick aluminum foil and placed in dry oven and sterilized for a period of not less than three hours at a temperature of 140-160 °C. Media and plastic ware cannot be sterilized by this method.
2. Wet heat:
The most popular method of sterilization both equipment and media is autoclaving at 121°C with a pressure of 15 psi (pounds per square inch) for 15 min (1.02 kg/cm2). Modern autoclaves are capable of providing saturated steam treatment ranging from 70-132 °C, which is a pressure of up to 25 psi. All the vessels containing medium should be placed vertically and should not be filled more than 40% to their total capacity.
3. Filter sterilization:
Filter sterilization or cold sterilization is used when a solution or medium cannot be sterilized by autoclaving. It is the property of the filter (porosity 0.22 to 0.45 µm) to retain the entire microorganism and make the solution free from microbes. This exclusion of microorganisms makes the solution sterilized without heating or autoclaving.
Only liquids can be sterilized by this method and not the plant materials or other things. This is commonly used where thermolabile or heat sensitive chemicals like plant growth regulators (IAA, GA3, Zeatin, Abscisic Acid), some bio-chemicals, enzymes for protoplasts isolation, antibiotics or nutrient solution for specific experiment are used to avoid decomposition during autoclaving. After sterilization, solution is added to the medium. In case of static medium, compound is added before solidification (cooling) after autoclaving.
Glass and membrane filters (nitro-cellulose membranes) are commercially available. Glass (sintered glass filters, Borosil) are available in different grades (G-1 to 5) and the G-5 (1-2 pm) is used for bacteriological purposes (Fig. 25.14). These filters have a porous glass disc for filtration of liquids and gases as a filter media which is non-corrosive and reusable.
The grades are classified by maximum pore size which is obtained by measuring the pressure at which the first air bubble breaks away from filter under certain conditions. The pressure differential is then used to calculate the equivalent capillary diameters in microns. The desired pore size is obtained by suitably controlling the grain size, firing time and temperature and the thickness of the disc. These filters are resistant to heat, solvents and detergents.
Membrane type filters (of different diameter) and porosity (0.22 to 0.45 µm) are available as disposable or reusable filters, or as filter holders containing a filter disc (Fig. 25.14). These filters can be connected through tubing or syringe to pass solution or gases. Filters are wrapped in aluminum foil or paper, autoclaved at 121°C for 15 min and then taken to laminar air flow bench. Solution is placed in funnel (glass type filter) or syringe (membrane type filter) and solution is allowed to pass through filter under suction (glass) or pressure (membrane).
A membrane filter or glass tube, filled with cotton may be placed between vacuum flask and suction pump to avoid entry of air from the outside during operation. Sterilized filtrate is collected in flask and dispensed in the liquid or molten medium with the help of sterilized pipette fitted with cotton plug. Volume added to the medium is adjusted to arrive at a final concentration. Membrane type filters are also used at inlets and outlets of various types including air, in a bioreactor system and to draw samples.
There are a variety of wet and dry heat treatments, radiations, filtration and gas and chemical treatments available for direct sterilization of material. Gas treatments are rarely used in the laboratory. Ultraviolet light treatment of working surfaces and sterile rooms are used in the labs while gamma radiation is used in the industry for the preparation of pre-sterilized disposable plastic-wares.
Sterilization of Tools:
Surgical blades and scalpels are not sterilized by dry heat because the high temperature makes the cutting edge dull. Such articles including spatula and forceps are usually immersed in 80% v/v (volume by volume) ethyl alcohol until required, and sterilized during use by frequent immersion in alcohol and flaming. Nowadays, bead sterilizer (works on principle of dry heat) is available for sterilizing such tools.
After an initial stabilization time of 30 minutes during which equipment attains a temperature of about 250 °C, units ensure total sterilization by destruction of all micro-organisms within seconds. This unit can conveniently placed on Laminar air-flow bench.
Uses of fumigation (formaldehyde, sulphur etc.) have been discouraged. It is used some times to clear the laboratory or working area from microorganisms. The place is not used for 3-4 days following fumigation. Culture rooms are fumigated only after removal of cultures. Generally, wiping of working places with 80% alcohol is sufficient for regular use.
13. Medium and Its Preparation:
The media used by earlier workers were based on Knop’s solution. Subsequently media developed by White (1943) and Heller (1953) were used. Murashige and Skoog’s medium (Murashige and Skoog, 1962) is a land mark in plant tissue culture research and is the most frequently used medium for all types of tissue culture work. Based on its composition, other media were evolved to meet the diverse experimental and species specific requirement.
There are – Linsmaier and Skoog (1965), B5 medium of Gamborg et. al. (1968), SH medium of Schenk and Hildebrandt (1972), Nitsch and Nitsch (1969) medium, and woody plant medium (WPM) of Llyod and McCown, (1980).
The methodology of plant tissue culture has advanced to the stage, where tissues from virtually any plant species can be cultured successfully. The successful plant tissue culture depends upon the choice of nutrient medium. The cells of most plant species can be grown on completely defined media. All the media consist of mineral salts, a carbon source (generally sucrose), vitamins and growth regulators. The MS medium designed for tobacco is now used widely for various species, in callus and cell cultures.
Medium Composition:
The nutrient medium for most plant tissue cultures is comprised of five groups of ingredients – inorganic nutrients, carbon source, vitamins, growth regulators and organic supplements.
Inorganic Nutrients:
Inorganic nutrients consist of macro- and micro-elements as their salts. Usually nutrient media contain 25 mM each of nitrate and potassium. For regular culture and cell cultures, the combined nitrogen level (nitrate and ammonium nitrogen) may reach up to 60 mM.
Ammonium is essential for most cultures but in lower concentrations than that of nitrate nitrogen. A concentration of 1-3 mM of calcium, magnesium and sulphate, is always adequate. The required micronutrients include I, B, Mn, Zn, Mo, Cu, Co and Fe. Role of different nutrients is given in the Table 25.2.
Carbon Source:
Glucose, fructose, maltose or sucrose (2-4%) can be used as source of energy or carbon but sucrose is the preferred source for most of the cultures. The sucrose in the medium is rapidly converted into glucose and fructose. The glucose is absorbed first followed by fructose.
Vitamins and amino acids:
Thiamine, pyridoxine and nicotinic acid are commonly used as vitamins in B5 and MS media. The former is required for most cultures while latter two promote cell growth. Amino acids and organic supplements -Amino acids serve as source of reduced nitrogen. In case of inadequate nitrogen, complex organic nitrogen supplement like casein hydrolysate (0.1-1 g/I) may be supplemented. Glycine is commonly used amino acid. Other organic supplements are coconut milk, yeast extract, peptone and malt extract. However, synthetic media are preferred and organic supplement of unknown chemical nature is used only when it is essential.
Plant Growth Regulators (PGR):
A balanced combination of PGR is required for sustained growth. Two types of combinations are used; one for cell proliferation consisting of (preferably) 2,4- dichlorophenoxy acetic acid (2,4-D) or 1-naphthalene acetic acid ( NAA) and a cytokinin (kinetin, benzyl adenosine, 2-isopentyladencisine, zeatin, thidiazuron), another for regeneration essentially containing low auxiti [(NAA, IAA, Indole butyric acid (IBA)] and a cytokinin in high amount, but not 2,4-D as an auxin. 2,4-D is known to induce cell proliferation but suppresses differentiation in dicot plants. However, 2,4-D and 2,4,5-T (2,4,5-trichlorophenoxy acetic acid) are effective in inducing somatic embryogenesis in cereal (monocots) and herbaceous dicot cultures.
Medium Preparation:
A convenient approach to prepare a medium is to have stock solutions of all the nutrients in a 10x or 50x concentration. Medium is prepared by suitably diluting the appropriate amount of stock solutions for desired volume of the medium. It may be advantageous to have separate stock solutions of calcium salt and potassium iodide.
All the ingredients are mixed, sugar added and pH is adjusted to 5.8-6.0 and medium is poured in the culture vessels. All the vessels are plugged with non-absorbent cotton, covered with aluminum foil and autoclaved at 121 °C for 15 min.
Prepared media can be stored for a few weeks before inoculation. Liquid medium for a given material is same as static medium used for callus cultures except gelling agent- agar. All the ingredients should be thoroughly mixed before dispensing in the vessels.




![image_thumb5[1] image_thumb5[1]](https://www.biologydiscussion.com/wp-content/uploads/2015/10/image_thumb51_thumb.png)