ADVERTISEMENTS:
Read this article to learn about the plant tissue culture. Its benefits, structure, types, techniques and applications.
Plant Tissue Culture:
Plant tissue culture broadly refers to the in vitro cultivation of plants, seeds and various parts of the plants (organs, embryos, tissues, single cells, protoplasts).
The cultivation process is invariably carried out in a nutrient culture medium under aseptic conditions.
ADVERTISEMENTS:
Plant cells have certain advantages over animal cells in culture systems. Unlike animal cells, highly mature and differentiated plant cells retain the ability of totipotency i.e. the ability of change to meristematic state and differentiate into a whole plant.
Benefits of Plant Tissue Culture:
Plant tissue culture is one of the most rapidly growing areas of biotechnology because of its high potential to develop improved crops and ornamental plants. With the advances made in the tissue culture technology, it is now possible to regenerate species of any plant in the laboratory.
To achieve the target of creating a new plant or a plant with desired characteristics, tissue culture is often coupled with recombinant DNA technology. The techniques of plant tissue culture have largely helped in the green revolution by improving the crop yield and quality.
The knowledge obtained from plant tissue cultures has contributed to our understanding of metabolism, growth, differentiation and morphogenesis of plant cells. Further, developments in tissue culture have helped to produce several pathogen-free plants, besides the synthesis of many biologically important compounds, including pharmaceuticals. Because of the wide range of applications, plant tissue culture attracts the attention of molecular biologists, plant breeders and industrialists.
Basic Structure and Growth of a Plant:
ADVERTISEMENTS:
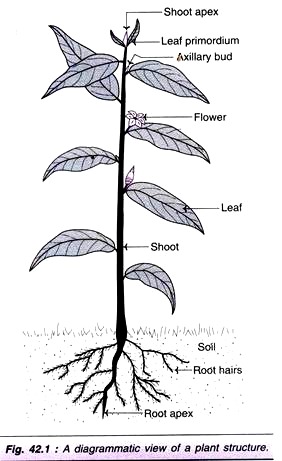
An adult plant basically consists of a stem and a root, each with many branches (Fig. 42.1). Both the stem and root are characterized by the presence of apical growth regions which are composed of meristematic cells. These cells are the primary source for all the cell types of a plant.
The plant growth and development occur in two different ways:
1. Determinate growth:
This is characterized by ceasation of growth as the plant parts attain certain size and shape, e.g., leaves, flowers, fruits.
2. Indeterminate growth:
This refers to the continuous growth of roots and stems under suitable conditions. It is possible due to the presence of meristems (in stems and roots) which can proliferate continuously. As the seed germinates and seedling emerges, the meristematic cells of the root apex multiply. Above the root apex, the cells grow in length without multiplication.
Some of the elongated cells of the outer layer develop into root hairs to absorb water and nutrients from the soil. As the plant grows, root cells differentiate into phloem and xylem. Phloem is responsible for the absorption of nutrients while xylem absorbs water.
The meristematic cells of the shoot apex divide leading to the growth of stem. Some of the stem cells differentiate and develop into leaf primordia, and then leaves. Axillary buds present between the leaf primordia and elongated stem also possess meristems which can multiply and give rise to branches and flowers.
A diagrammatic view of a plant and a flower are respectively depicted in Fig. 42.1 and Fig. 42.2.
Conventional Plant Breeding and Plant Tissue Culture:
Since the time immemorial, man has been closely involved in the improvement of plants to meet his basic needs. The conventional methods employed for crop improvement are very tedious and longtime processes (sometimes decades). Further, in the conventional breeding methods, it is not possible to introduce desired genes to generate new characters or products.
With the developments in plant tissue culture, it is now possible to reduce the time for the creation of new plants with desired characteristics, transfer of new genes into plant cells and large scale production of commercially important products.
Terms Used in Tissue Culture:
ADVERTISEMENTS:
A selected list of the most commonly used terms in tissue culture are briefly explained
Explant:
An excised piece of differentiated tissue or organ is regarded as an explant. The explant may be taken from any part of the plant body e.g., leaf, stem, root.
Callus:
ADVERTISEMENTS:
The unorganized and undifferentiated mass of plant cells is referred to as callus. Generally, when plant cells are cultured in a suitable medium, they divide to form callus i.e., a mass of parenchymatous cells.
Dedifferentiation:
The phenomenon of mature cells reverting to meristematic state to produce callus is dedifferentiation. Dedifferentiation is possible since the non- dividing quiescent cells of the explant, when grown in a suitable culture medium revert to meristematic state.
Re-differentiation:
ADVERTISEMENTS:
The ability of the callus cells to differentiate into a plant organ or a whole plant is regarded as re-differentiation.
Totipotency:
The ability of an individual cell to develop into a whole plant is referred to as cellular totipotency. The inherent characteristic features of plant cells namely dedifferentiation and re-differentiation are responsible for the phenomenon of totipotency. The other terms used in plant tissue culture are explained at appropriate places.
Brief History of Plant Tissue Culture:
About 250 years ago (1756), Henri-Louis Duhamel du Monceau demonstrated callus formation on the decorticated regions of elm plants. Many botanists regard this work as the forward for the discovery of plant tissue culture. In 1853, Trecul published pictures of callus formation in plants.
German botanist Gottlieb Haberlandt (1902), regarded as the father of plant tissue culture, first developed the concept of in vitro cell culture. He was the first to culture isolated and fully differentiated plant cells in a nutrient medium. During 1934-1940, three scientists namely Gautheret, White and Nobecourt largely contributed to the developments made in plant tissue culture.
Good progress and rapid developments occurred after 1940 in plant tissue culture techniques. Steward and Reinert (1959) first discovered somatic embryo production in vitro. Maheswari and Guha (1964) from India were the first to develop anther culture and poller culture for the production of haploid plants.
ADVERTISEMENTS:
Types of Culture:
There are different types of plant tissue culture techniques, mainly based on the explant used (Fig. 42.3).
Callus culture:
This involves the culture of differentiated tissue from explant which dedifferentiates in vitro to form callus.
Organ culture:
ADVERTISEMENTS:
Culture of isolated plant organs is referred to as organ culture. The organ used may be embryo, seed, root, endosperm, anther, ovary, ovule, meristem (shoot tip) or nucellus. The organ culture may be organized or unorganized.
Organized organ culture:
When a well-organized structure of a plant (seed, embryo) is used in culture, it is referred to as organized culture. In this type of culture, the characteristic individual organ structure is maintained and the progeny formed is similar in structure as that of the original organ.
Unorganized organ culture:
This involves the isolation of cells or tissues of a part of the organ, and their culture in vitro. Unorganized culture results in the formation of callus. The callus can be dispersed into aggregates of cells and/or single cells to give a suspension culture.
Cell culture:
The culture of isolated individual cells, obtained from an explant tissue or callus is regarded as cell culture. These cultures are carried out in dispension medium and are referred to as cell suspension cultures.
Protoplast culture:
Plant protoplasts (i.e., cells devoid of cell walls) are also used in the laboratory for culture.
Basic Technique of Plant Tissue Culture:
The general procedure adopted for isolation and culture of plant tissues is depicted in Fig. 42.4
The requisite explants (buds, stem, seeds) are trimmed and then subjected to sterilization in a detergent solution. After washing in sterile distilled water, the explants are placed in a suitable culture medium (liquid or semisolid form) and incubated. This results in the establishment of culture. The mother cultures can be subdivided, as frequently as needed, to give daughter cultures.
The most important aspect of in vitro culture technique is to carry out all the operations under aseptic conditions. Bacteria and fungi are the most common contaminants in plant tissue culture. They grow much faster in culture and often kill the plant tissue.
Further, the contaminants also produce certain compounds which are toxic to the plant tissue. Therefore, it is absolutely essential that aseptic conditions are maintained throughout the tissue culture operations. Some of the culture techniques are described here while a few others are discussed at appropriate places.
Applications of Plant Tissue Cultures:
Plant tissue cultures are associated with a wide range of applications—the most important being the production of pharmaceutical, medicinal and other industrially important compounds.
In addition, tissue cultures are useful for several other purposes listed below:
1. To study the respiration and metabolism of plants.
2. For the evaluation of organ functions in plants.
3. To study the various plant diseases and work out methods-for their elimination.
4. Single cell clones are useful for genetic, morphological and pathological studies.
5. Embryonic cell suspensions can be used for large scale clonal propagation.
6. Somatic embryos from cell suspensions can be stored for long term in germplasm banks.
7. In the production of variant clones with new characteristics, a phenomenon referred to as soma clonal variations.
8. Production of haploids (with a single set of chromosomes) for improving crops.
9. Mutant cells can be selected from cultures and used for crop improvement.
10. Immature embryos can be cultured in vitro to produce hybrids, a process referred to as embryo rescue.
Callus Culture:
Callus is the undifferentiated and unorganized mass of plant cells. It is basically a tumor tissue which usually forms on wounds of differentiated tissues or organs. Callus cells are parenchymatous in nature although not truly homogenous. On careful examination, callus is found to contain some quantity of differentiated tissue, besides the bulk of non-differentiated tissue.
Callus formation in vivo is frequently observed as a result of wounds at the cut edges of stems or roots. Invasion of microorganisms or damage by insect feeding usually occurs through callus. An outline of technique used for callus culture, and initiation of suspension culture is depicted in Fig. 42.5.
Explants for callus culture:
The starting materials (explates) for callus culture may be the differentiated tissue from any part of the plant (root, stem, leaf, anther, flower etc.). The selected explant tissues may be at different stages of cell division, cell proliferation and organization into different distinct specialized structures. If the explant used possesses meristematic cells, then the cell division and multiplication will be rapid.
Factors Affecting Callus Culture:
Many factors are known to influence callus formation in vitro culture. These include the source of the explant and its genotype, composition of the medium (MS medium most commonly used), physical factors (temperature, light etc.) and growth factors. Other important factors affecting callus culture are — age of the plant, location of explant, physiology and growth conditions of the plant.
Physical factors:
A temperature in the range of 22-28°C is suitable for adequate callus formation. As regards the effect of light on callus, it is largely dependent on the plant species-light may be essential for some plants while darkness is required by others.
Growth regulators:
The growth regulators to the medium strongly influence callus formation. Based on the nature of the explant and its genotype, and the endogenous content of the hormone, the requirements of growth regulators may be categorized into 3 groups
1. Auxin alone
2. Cytokinin alone
3. Both auxin and cytokinin.
Suspension culture from callus:
Suspension cultures can be initiated by transferring friable callus to liquid nutrient medium (Fig. 42.5). As the medium is liquid in nature, the pieces of callus remain submerged. This creates anaerobic condition and ultimately the cells may die. For this reason, suspension cultures have to be agitated by a rotary shaker. Due to agitation, the cells gets dispersed, besides their exposure to aeration.
Applications of Callus Cultures:
Callus cultures are slow-growth plant culture systems in static medium. This enables to conduct several studies related to many aspects of plants (growth, differentiation and metabolism) as listed below.
i. Nutritional requirements of plants.
ii. Cell and organ differentiation.
iii. Development of suspension and protoplast cultures.
iv. Somaclonal variations.
v. Genetic transformations.
vi. Production of secondary metabolites and their regulation.
Cell Culture:
The first attempt to culture single cells (obtained from leaves of flowering plants) was made in as early as 1902 by Haberlandt. Although he was unsuccessful to achieve cell division in vitro, his work gave a stimulus to several researchers. In later years, good success was achieved not only for cell division but also to raise complete plants from single cell cultures.
Applications of Cell Cultures:
Cultured cells have a wide range of applications in biology.
1. Elucidation of the pathways of cellular metabolism.
2. Serve as good targets for mutation and selection of desirable mutants.
3. Production of secondary metabolites of commercial interest.
4. Good potential for crop improvement.
Cell Culture Technique:
The in vitro cell culture technique broadly involves the following aspects:
1. Isolation of single cells.
2. Suspension cultures growth and sub-culturing.
3. Types of suspension cultures.
4. Synchronization of suspension cultures.
5. Measurement of growth of cultures.
6. Measurement of viability of cultured cells.
The salient features of the above steps are briefly described.
1. Isolation of Single Cells:
The cells employed for in vitro culture may be obtained from plant organs, and from cultured tissues.
From plant organs:
Plant leaves with homogenous population of cells are the ideal sources for cell culture. Single cells can be isolated from leaves by mechanical or enzymatic methods.
Mechanical method:
Surface sterilized leaves are cut into small pieces (< 1 cm2), suspended in a medium and subjected to grinding in a glass homogenizer tube. The homogenate is filtered through filters and then centrifuged at a low speed to remove the cellular debris. The supernatant is removed and diluted to achieve the required cell density.
Enzymatic method:
The enzyme macerozyme (under suitable osmotic pressure) can release the individual cells from the leaf tissues. Macerozyme degrades middle lamella and cell walls of parenchymatous tissues.
From cultured tissues:
Single cells can be isolated from callus cultures (grown from cut pieces of surface sterilized plant parts). Repeated sub-culturing of callus on agar medium improves the friability of callus so that fine cell suspensions are obtained.
2. Suspension Cultures — Growth and Subculture:
The isolated cells are grown in suspension cultures. Cell suspensions are maintained by routine sub-culturing in a fresh medium. For this purpose, the cells are picked up in the early stationary phase and transferred. As the cells are incubated in suspension cultures, the cells divide and enlarge.
The incubation period is dependent on:
i. Initial cell density
ii. Duration of lag phase
iii. Growth rate of cells.
Among these, cell density is very crucial. The initial cell density used in the subcultures is very critical, and largely depends on the type of suspension culture being maintained. With low initial cell densities, the lag phase and log phases of growth get prolonged.
Whenever a new suspension culture is started, it is necessary to determine the optical cell density in relation the volume of culture medium, so that maximum cell growth can be achieved. With low cell densities, the culture will not grow well, and requires additional supplementation of metabolites to the medium. The normal incubation time for the suspension cultures is in the range of 21-28 days.
3. Types of Suspension Cultures:
There are mainly two types of suspension cultures — batch cultures and continuous cultures.
Batch cultures:
A batch culture is a cell suspension culture grown in a fixed volume of nutrient culture medium. In batch culture, cell division and cell growth coupled with increase in biomass occur until one of the factors in the culture environment (nutrient, O2 supply) becomes limiting. The cells exhibit the following five phases of growth when the cell number in suspension cultures is plotted against the time of incubation (Fig. 42.6).
1. Lag phase characterized by preparation of cells to divide.
2. Log phase (exponential phase) where the rate of cell multiplication is highest.
3. Linear phase represented by slowness in cell division and increase in cell size expansion.
4. Deceleration phase characterized by decrease in cell division and cell expansion.
5. Stationary phase represented by a constant number of cells and their size.
The batch cultures can be maintained continuously by transferring small amounts of the suspension medium (with inoculum) to fresh medium at regular intervals (2-3 days). Batch cultures are characterized by a constant change in the pattern of cell growth and metabolism. For this reason, these cultures are not ideally suited for the studies related to cellular behaviour.
Continuous cultures:
In continuous cultures, there is a regular addition of fresh nutrient medium and draining out the used medium so that the culture volume is normally constant. These cultures are carried out in specially designed culture vessels (bioreactors).
Continuous cultures are carried out under defined and controlled conditions—cell density, nutrients, O2, pH etc. The cells in these cultures are mostly at an exponential phase (log phase) of growth.
Continuous cultures are of two types—open and closed.
Open continuous cultures:
In these cultures, the inflow of fresh medium is balanced with the outflow of the volume of spent medium along with the cells. The addition of fresh medium and culture harvest are so adjusted that the cultures are maintained indefinitely at a constant growth rate. At a steady state, the rate of cells removed from the cultures equals to the rate of formation of new cells.
Open continuous culture system is regarded as chemostat if the cellular growth rate and density are kept constant by limiting a nutrient in the medium (glucose, nitrogen, phosphorus). In chemostat cultures, except the limiting nutrient, all other nutrients are kept at higher concentrations. As a result, any increase or decrease in the limiting nutrient will correspondingly increase or decrease the growth rate of cells.
In turbidostat open continuous cultures, addition of fresh medium is done whenever there is an increase in turbidity so that the suspension culture system is maintained at a fixed optical density. Thus, in these culture systems, turbidity is preselected on the basis of biomass density in cultures, and they are maintained by intermittent addition of medium and washout of cells.
Closed continuous cultures:
In these cultures, the cells are retained while the inflow of fresh medium is balanced with the outflow of corresponding spent medium. The cells present in the outflowing medium are separated (mechanically) and added back to the culture system. As a result, there is a continuous increase in the biomass in closed continuous cultures. These cultures are useful for studies related to cytodifferentiation, and for the production of certain secondary metabolites e.g., polysaccharides, coumarins.
4. Synchronization of Suspension Cultures:
In the normal circumstances, the cultured plant cells vary greatly in size, shape, cell cycle etc., and are said to be asynchronous. Due to variations in the cells, they are not suitable for genetic, biochemical and physiological studies. For these reasons, synchronization of cells assumes significance.
Synchronization of cultured cells broadly refers to the organized existence of majority of cells in the same cell cycle phase simultaneously.
A synchronous culture may be regarded as a culture in which the cell cycles or specific phase of cycles for majority of cultured cells occurs simultaneously.
Several methods are in use to bring out synchronization of suspension cultures. They may be broadly divided into physical and chemical methods.
Physical methods:
The environmental culture growth influencing physical parameters (light, temperature) and the physical properties of the cell (size) can be carefully monitored to achieve reasonably good degree of synchronization. A couple of them are described
Cold treatment:
When the suspension cultures are subjected to low temperature (around 4°C) shock synchronization occurs. Cold treatment in combination with nutrient starvation gives better results.
Selection by volume:
The cells in suspension culture can be selected based on the size of the aggregates, and by this approach, cell synchronization can be achieved.
Chemical methods:
The chemical methods for synchronization of suspension cultures include the use of chemical inhibitors, and deprivation of an essential growth factor (nutrient starvation). By this approach, the cell cycle can be arrested at a particular stage, and then allowed to occur simultaneously so that synchronization is achieved.
Chemical inhibition:
Inhibitors of DNA synthesis (5-amino uracil, hydroxyurea, 5-fluorodeoxypurine), when added to the cultures results in the accumulation of cells at G1 phase. And on removal of the inhibitor, synchronization of cell division occurs.
Colchicine is a strong inhibitor to arrest the growth of cells at metaphase. It inhibits spindle formation during the metaphase stage of cell division. Exposure to colchicine must be done for a short period (during the exponential growth phase), as long duration exposure may lead to mitoses.
Starvation:
When an essential nutrient or growth promoting compound is deprived in suspension cultures, this results in stationary growth phase. On supplementation of the missing nutrient compound, cell growth resumption occurs synchronously. Some workers have reported that deprivation and subsequent addition of growth hormone also induces synchronization of cell cultures.
5. Measurement of Growth of Cultures:
It is necessary to assess the growth of cells in cultures. The parameters selected for the measuring growth of suspension cultures include cell counting, packed cell volume and weight increase.
Cell counting:
Although cell counting to assess culture growth is reasonably accurate, it is tedious and time consuming. This is because cells in suspension culture mostly exist as colonies in varying sizes. These cells have to be first disrupted (by treating with pectinase or chromic acid), separated, and then counted using a haemocytometer.
Packed cell volume:
Packed cell volume (PCV) is expressed as ml of pellet per ml of culture. To determine PCV, a measured volume of suspension culture is centrifuged (usually at 2000 x g for 5 minutes) and the volume of the pellet or packed cell volume is recorded. After centrifugation the supernatant can be discarded, the pellet washed, dried overnight and weighed. This gives cell dry weight.
Cell fresh weight:
The wet cells are collected on a pre-weighed nylon fabric filter (supported in funnel). They are washed to remove the medium, drained under vacuum and weighed. This gives the fresh weight of cells. However, large samples have to be used for accurate weights.
6. Measurement of Viability of Cultured Cells:
The viability of cells is the most important factor for the growth of cells. Viability of cultured cells can be measured by microscopic examination of cells directly or after staining them.
Phase contrast microscopy:
The viable cells can be detected by the presence of healthy nuclei. Phase contrast microscope is used for this purpose.
Evan’s blue staining:
A dilute solution of Evan’s blue (0.025% w/v) dye stains the dead or damaged cells while the living (viable) cells remain unstained.
Fluorescein diacetate method:
When the cell suspension is incubated with fluorescein diacetate (FDA) at a final concentration of 0.01%, it is cleaved by esterase enzyme of living cells. As a result, the polar portion of fluorescein which emits green fluorescence under ultraviolet (UV) light is released. The viable cells can be detected by their fluorescence, since fluorescein accumulates in the living cells only.
Culture of Isolated Single Cells (Single Cell Clones):
A clone is a mass of cells, all of them derived through mitosis from a single cell. The cells of the clone are expected to be identical with regard to genotype and karyotype. However, changes in these cells may occur after cloning. Single cells separated from plant tissues under suitable conditions can form clones.
Single cells can be cultured by the following methods:
1. Filter paper raft-nurse tissue technique
2. Micro-chamber technique
3. Micro-drop method
4. Bergman’s plating technique.
Filter paper raft-nurse tissue technique:
Small pieces of sterile filter papers are placed on established callus cultures several days before the start of single cell culture. Single cell is now placed on the filter paper (Fig. 42.7A). This filter paper, wetted by the exudates from callus tissue (by diffusion) supplies the nutrients to the single cell. The cell divides and forms clones on the filter paper. These colonies can be isolated and cultured.
Micro-chamber technique:
A microscopic slide or a coverslip can be used to create a micro-chamber. Sometimes, a cavity slide can be directly used. A drop of the medium containing a single cell is placed in the micro-chamber. A drop of mineral oil is placed on either side of the culture drop which is covered with a coverslip (Fig. 42.7B). On incubation, single cell colonies are formed.
Micro-drop method:
For the culture of single cells by micro-drop method, a specially designed dish (cuprak dish) is used. It has a small outer chamber (to be filled with sterile distilled water) and a large inner chamber with a number of micro-wells (Fig. 42.7C). The cell density of the medium is adjusted in such a way that it contains one cell per droplet.
Bergmann’s plating technique:
Bergmann (1960) developed a technique for cloning of single cells. Now a days, Bergmann’s plating technique is the most widely used method for culture of isolated single cells. This method is depicted in Fig. 42.8 and briefly described hereunder.
The cell suspension is filtered through a sieve to obtain single cells in the filtrate. The free cells are suspended in a liquid medium, at a density twice than the required density for cell plating. Now, equal volumes of melted agar (30-35°C) and medium containing cells are mixed.
The agar medium with single cells is poured and spread out in a petridish so that the cells are evenly distributed on a thin layer (of agar after it solidifies). The petridishes (culture dishes) are sealed with a parafilm and incubated at 25°C in dark or diffused light. The single cells divide and develop into clones. The viability of cells in single clones can be measured by the same techniques that have been described for suspension cultures.