ADVERTISEMENTS:
Read this article to learn about the basic plant tissue culture:
(1) Culture Initiation (2) Callus Culture (3) Cell Suspension Culture (4) Time Course of Growth (5) Single Cell Cultures and (6) Cloning and Selection of Cells.
Thing # 1. Culture Initiation:
Plant parts are used to initiate and establish in vitro (in glass) growing cultures. In comparison to in vitro grown cultures, in case of field or pot grown plants, growth is affected by various soil and environmental factors of unknown nature.
ADVERTISEMENTS:
Therefore, it is difficult to study the factors affecting the growth, differentiation and metabolism of the plant.
Plant tissue culture provides an excellent system to study the plant growth and differentiation in totally controlled conditions. Cultures are grown in a completely defined chemical and physical environment. Thus, by changing the factors, one by one, the influence of a particular factor can be determined very easily. The field grown (in vivo) plants are autotrophic, i.e., they synthesize their own food by photosynthesis.
In contrast to this, sugar is essential in the medium for the growth of in vitro cultures, i.e., they are unable to synthesize their carbohydrate requirement (even though cultures are grown in light, photosynthesis is almost negligible).
Explant Type:
The present knowledge permits the use of any plant part as a source of material to initiate cultures. The plant part used for this purpose is known as an ‘Explant.’ Nodal and inter nodal segments of stem, apical and axillary bud, leaf, leaf disc, petiole, anther, pollen, flower bud, petal, inflorescence, ovule, ovary root and even isolated epidermal peel, gland and trichome have been used as an explants.
Preparation of Explants:
ADVERTISEMENTS:
In vivo material, when brought to laboratory, it is always preferable to bring material under ice/dry ice to minimize catabolic activity. Material is washed thoroughly with tap water and liquid detergent (e.g., Tween-80), and then washed thoroughly. After proper cleaning and removal of adhering particles, material is sterilized and used.
Some hard seeds (like leguminous and hard seed coat seeds) may require pretreatment (2-10 min) with 50% sulphuric acid to break seed coat dormancy. This should be done with care and seeds should be washed thoroughly after acid treatment under running tape water for 1-2 hours.
When axillary or apical buds are used, extra leaves are removed, and pre-treated with a wetting agent (quick dip in 70% ethanol or 5-10 min in detergent solution) to facilitate penetration of disinfectant.
Explants are prepared in suitable size for the purpose of sterilization, inoculation and suitability to generate culture. Stem segments consist of single node or multiple nodes, or inter nodes are used while leaves can be used as whole leaf or leaf disc can be prepared.
When tuberous materials like Jerusalem artichoke (Helianthus tuberosus), sugar beet roots (Beta vulagaris) or carrot roots (Daucus carota) are used as an explant, large pieces/complete tubers are sterilized and then with the help of scalpel and cork borer, tissue cylinders and discs from cylinders are prepared under aseptic conditions. The explant preparation and sterilization are carried out under laminar flow bench. Sterilization of explants is already described in the previous chapter using sodium hypochlorite or mercuric chloride.
Thing # 2. Callus Culture:
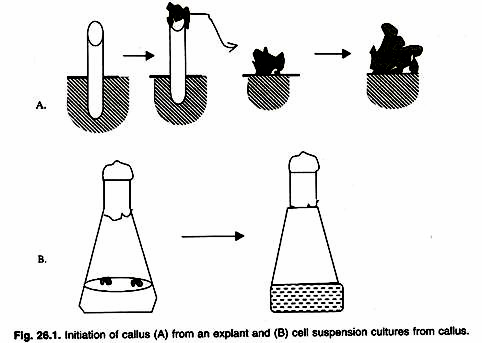
Cell from any plant species can be cultured aseptically on or in a nutrient medium. The cultures are initiated by planting a sterilized tissue (an explant) on an agar medium. Within 2-4 weeks, depending upon plant species, a mass of unorganized cells (callus) is produced. Such a callus can be sub-cultured indefinitely by transferring a small piece on to the fresh agar medium. When a suitable sized and sterilized explant is transferred aseptically under a laminar flow bench on to an appropriate nutrient medium (for example, MS medium or B5 medium) containing appropriate combination of plant growth regulators, it produces callus (Fig. 26.1).
The callus is produced from outer layers of cortical cells in a stem explant by repetitive division of the cells. These dividing cells generate pressure on the epidermis, which ultimately ruptures exposing newly formed callus. By continuous division of cells produces a mass of cells or callus on the explant. This callus is separated from the explants and transferred on to fresh medium.
After attaining growth, callus is subcultures at regular interval of 3 to 4 weeks by dividing it in to pieces (250 to 500 mg each) and transferred on to fresh medium. The callus piece used to inoculate a flask is known as ‘inoculum’. Inocula (plural of inoculum) of similar shape and size are used in all the subcultures and experiments.
Callus cultures are slow growing system as compared to cell suspension cultures (Table 26.1). Cells grow as clumps or masses in callus cultures and only lower cells are in contact with the medium whereas, cells in upper layers get their nutrients from cells in lower layers. Cells are in close association as compared to cell cultures in which all the cells are in direct contact with the medium and dissociated.
Application of Callus Cultures:
ADVERTISEMENTS:
Callus cultures are slow growing system on static medium and offers a unique system (as compared to in vivo grown plants) to study the following aspects of plant metabolism and differentiation:
1. Nutrition of plants.
2. Cell and organ differentiation and morphogenesis.
ADVERTISEMENTS:
3. Somaclonal variation and its exploitation.
4. Developing cell suspension cultures and protoplasts cultures.
5. Genetic transformation using ballastic particle gun technology.
6. In the production of secondary metabolites and their regulation.
Thing # 3. Cell Suspension Culture:
Cell suspension cultures are initiated by transferring friable callus to liquid nutrient medium. In liquid medium, plant tissues remains submerged which leads to anaerobic conditions and ultimately death of the cells. Therefore, such cultures are agitated on rotary shaker at 80-150 rpm, with an orbital diameter of 2.5-5.0 cms.
ADVERTISEMENTS:
Agitation serves both to aerate the cultures and to disperse the cells. Cells from the inoculum are separated during this process and a suspension of cells is produced. The division rates of suspension culture cells at the ex-potential phase are typically higher than callus cells, but doubling times are slow than bacterial cells and usually vary from 24- 72 hrs.
It is a common observation that if relatively small number cells are transferred (low inoculum density) to a new medium (either static or liquid), they may fail to divide; whereas a larger quantity of tissue transferred from the same culture may proliferate rapidly on the same medium.
This observation has led to the concept of ‘critical initial cell density. This is defined as the smallest inoculum per volume of medium, from which a new culture can be reproducibly grown. There are a few conditions, which determine the critical initial density of cells.
ADVERTISEMENTS:
These are:
(1) The cultures physiological characteristic.
(2) The length of time and conditions under which the culture was previously maintained.
(3) The composition of the fresh medium.
The third point is of interest. As the isolated cells are failed to grow on fresh medium, ‘conditioned medium’ or ‘nurse tissue’ conditions are used to grow isolated cells or protoplasts. A ‘conditioned medium’ is the medium on which some tissues were previously grown. This makes the minor adjustment in the nutrients and chemical substances released in the medium by the callus, promotes the growth of isolated cells of protoplasts.
In cell suspension cultures, cells grow as isolated single cell and cell aggregates of a few cells to a few hundred cells. Cells aggregation vary from species to species and sometimes it is difficult to maintain fine cell suspension culture, e.g., in members of the family Rutaceae.
ADVERTISEMENTS:
Cell cultures are sub-cultured by dilution of the stock culture, 5 to 10 times v/v (volume by volume), depending upon the growth of cells. As mentioned above, growth of cell suspension cultures is always higher than callus cultures and, therefore, required rapid subculture (7-21 days) as compared to callus cultures (4-8 weeks).
Cell cultures in the liquid medium are grown as:
(a) Batch cultures in shake flasks and bioreactor.
(b) Continuous culture system.
Batch Culture:
Whether cultures are grown in flasks (100 ml to 2 litres capacity containing 20 to 500 ml liquid medium) or in a bioreactor, system is used as a batch. Inoculated cultures are harvested after a definitive growth period (determined by growing cultures earlier and recording the growth phases during 1 to 6 weeks growth) at end of stationary phase and cultures are harvested for a specific objective. Such cultures are grown again and again in batches for the purpose of experiments and known as batch cultures.
Continuous Culture:
ADVERTISEMENTS:
Continuous cultures are usually grown in a bioreactor, (also known as chemostate) when inputs (nutrients, oxygen etc) and outputs (spent medium, cells) are precisely controlled and cells are grown under a defined conditions of nutrients, pH, oxygen and cell density continuously for the purpose of study.
Such cultures are known as continuous cultures. This type of work is possible only after basic study of growth and metabolism in small shake flasks. In continuous culture, cells are always in an exponential phase of growth.
Thing # 4. Time Course of Growth:
The growth of undifferentiated callus and suspension culture can be quantified in a number of ways, but most commonly as an increase in fresh weight, dry weight or cell numbers over a time course. Growth pattern of such cultures is always forming a sigmoid curve. This curve is consist of a lag phase, in which there is a little or no cell division, an exponential phase in which maximum cell growth occurs, followed by a linear and stationary phase, in which the proportion of dividing cells gradually declines (Fig. 26.3).
Each stage can be characterized by distinct structural and biochemical features but, infect whole growth cycle is a continuous physiological process. At any particular time a heterogeneous population of cells differing in morphological and physiological characters can be observed. Usually the cultures are sub-cultured at end of exponential phase or during stationary phase.
Fresh weight of callus is determined by carefully removing the adhering agar from callus and immediately weighing the callus in a sensitive balance. Cell cultures are filtered in a butcher funnel lined with filter paper, washed with distilled water (under mild vacuum) and weighed. Dry weights are determined by drying the cells to a constant weight at 60 °C in an oven and weighing in a balance.
Packed cell volume (PCV) and cell culture is determined by centrifuging it at 1000 rpm for 5 min in a graduated centrifuge tube and measuring the volume occupied by the cells out of total volume of the cell suspension. Cells are counted by a haemocytometer under compound microscope during growth period.
Thing # 5. Single Cell Cultures:
1. Nurse Tissue Culture:
Single cell cultures are obtained from established cell suspension cultures. Cell suspension cultures are comprised of mixture of single cells and small colonies of cells. These latter strains of cells are not necessarily derived from single cells. Thus, strains desired from such cultures are not true single cell clones.
Single cell clones are provided the means for pure culture studies for higher plants comparable to those used for microorganisms. Several methods are developed for obtaining single cell cultures and establishing single cell clones of higher plants.
The first successful higher plants single cell isolations were made by Muir and co-workers in 1954 at Wisconsin University, Madison, USA, using nurse tissue culture technique for marigold and tobacco callus cultures. Isolated single cells provide a unique system to study differentiation and divisions in a cell by monitoring changes under a microscope in a cell.
Nurse Tissue Culture Technique:
Single cells are observed and picked-up from cell suspension cultures under the microscope with the help of micropipette. Several days previous to the isolation of single cells, small sterile pieces (8×8 mm) of filter papers are places aseptically on the top of established callus of same or different species. By this time, filter paper place on nurse callus tissue is wetted by liquid and nutrients released by the cultures.
The single cell, then placed on the filter paper raft, may divide and produce a small colony of cells within a few days or weeks. This small colony of cells or microcalli can be transferred to fresh medium, where it survives (Fig. 26.4). A number of previously recalcitrant (difficult to grow in culture) species, notably monocotyledons such as rice and maize have been grown by such technique.
2. Cell Sieving:
Cell suspensions are filtered through sieves of varying mesh (nylon or stainless steel wire gauge of different porosity) depending upon the size of the cells and aggregates in the suspension culture. A series of 1-3 mesh (80-150 mesh) may be used to obtain cells of desired size and number (Fig. 26.5). A different fraction so obtained contains single cells and clumps of cells depending upon the mesh size. These fractions can be used directly for initiating cell suspensions or may be used after concentrating the fraction by centrifugation.
Fractions may be used to raise clones by plating the cells of these fractions. The appropriate fractions contain cells of similar shape, size and metabolic state. Such cultures are used to obtain synchronous cultures. Synchronous cultures are those cultures in which all the cells divide at the same time. Therefore, at one time all the cells divide and next time all the cells are in a stationary phase.
This time interval between two cycles of cell division is called ‘doubling time’ (Fig. 26.6). Such cultures are useful in developing embryonic cultures as embryos of similar age can be obtained, which is also a necessity for the production of seeds at large scale of a species.
Cell plating and cell sieving methods are alternative methods to obtain strains and clones of cells. Microscopic observations and precautions are necessary to ensure that a colony of cells is originating from a single cell. By this method a true single cell clone can be obtained. Cell plating and cell sieving methods have been extensively used to obtain a large number of single cells and their colonies. Bergman (1959-1960) was the pioneer in establishing the cell plating technique.
3. Micro-culture Chamber:
Another method of isolating and establishing single cell clones of higher plant cells is the micro-culture chamber method. This technique was developed in the laboratory of Prof. A.C. Hildebrandt at Wisconsin University, Madison, USA by Jones et al. (1960). For the study of single isolated cells with this method, the single cell is cultured aseptically in a drop of liquid medium surrounded by sterile mineral oil on a microscope slide. A mini chamber is prepared using three cover glasses as shown in the Figure 26.7.
The drop of medium containing cells is placed on a sterile microscope slide and ringed with sterile mineral oil. All the cover glasses are sealed with mineral (Paraffin) oil ensuring aseptic growth. This micro-chamber is placed in a sterile petridish and incubated. Cells grow in this system and can be observed periodically. Mineral oil prevents the water (medium) loss. Small colonies thus developed in chamber are aseptically transferred to fresh medium.
Applications of Cell Culture:
1. Cell culture offers enormous opportunities in the study of single cells and group of cells.
2. In the isolation of protoplasts.
3. In cell cloning by the plating technique with or without specific treatment, e.g., mutagens, amino acid analogues etc.
4. Development of cell lines for various types of resistance e.g., salt and drought, and toxin resistant lines.
5. In scale-up technology using bioreactors of various types.
6. In providing an excellent system of micro-propagation on mass-scale by somatic embryogenesis in cell culture and use of bioreactor. Such system can be used for artificial seed production.
7. In the study of nutrition.
8. In cellular differentiation, single cells constitute an excellent system to study cyto-differentiation in a cell leading to tracheiry element formation and plastids differentiation etc.
9. In secondary product formation, regulation and biosynthesis.
10. In the process of cell division and factors affecting cell divisions and related process.
Thing # 6. Cloning and Selection of Cells:
Plating Technique:
Clones of single cells or of cell aggregate origin are obtained by plating appropriate cultures. Single-cell clones can be obtained by isolating single cells or by isolating protoplasts. Therefore, in both cases a product of single cell is obtained which is supposed to be the best method for obtaining single-cell product.
This way a definite selection of producer cells can be made. Plant cells grow in suspension in units ranging from single cells to clusters of more than 1000 cells. Such cultures can be made uniform in cell size by filtration through screens of appropriate size (Sigma chemical Co., USA).
A screen of appropriate size is selected on the basis of size of the cells in the suspension. A uniform and defined, 1 ml suspension is spread over the surface of a 25 ml medium in 100 x 15 mm plastic (presterilized) petri dishes. The concentration of cells in the suspension determines the amount of inoculum.
Generally, 100 mg fresh weight is reasonable to conduct plating. Single cells, smaller or larger aggregates can be used for plating and selection, depending on the growth of species in the cell cultures (Fig. 26.8). Obtaining a suspension of single cells for plating has been usually achieved by filtration through a nylon net (150-250 µm). In practice, it is impossible to get a pure single cell fraction and mostly inoculum consists of single cells and 3-4 cell aggregates.
It is assumed that the aggregates are derived from single cells. By obtaining protoplasts, true single-cell clones can be obtained. Optimization of culture conditions is a prerequisite for inducing cell diversions in single-cell cultures.
Chemically defined cell culture media may not support cell division at low cell densities of less than 9000-15,000 cells per ml, a critical inoculum density. However, in order to achieve single cell clones, cells must be plated at low densities in order to prevent overlapping of the growing colonies. Use of conditioned medium in varying proportion with fresh medium has been suggested to achieve growth of single cells.
A conditioned medium is one used for the growth of cells previously. Once colonies are developed, they are grown separately by regular sub cultures of whole colony on fresh medium as deemed necessary for that species. When sufficient callus is produced (usually after 4-6 subcultures), half the callus is used for subculture and half for analysis of secondary metabolites.
Selected colonies (clones) are grown and their growth and primary/ secondary metabolite contents are determined. Low productive cells/clones are discarded. Quicker analytical methods are helpful in early selection of clones.
Cell Lines Selection for Disease Resistance:
Plant tissue culture provides a powerful technique to assist the plant breeder in improving the propagation and performance of agricultural, horticultural and forest species. Plants are affected by environmental stresses biotic (fungi, bacteria, nematodes and viruses etc) and abiotic (salinity, draught, toxic metals etc). Resistance to these pathogens may be monogenic or polygenic.
Generally, monogenic resistance is high. Plant cells are grown with a pathogen or pathogenic fungi (dual culture) or on a medium containing fungal toxins. Cells survive on such media are supposed to be resistant to fungi or its toxin. These cultures are regenerated and plants are again tested for resistance against the pathogens using standard pathological protocols.
Pearl millet cells were selected for downy mildew resistance (Sclerospora gramnicola) using dual culture techniques. Similarly, resistance cell lines of rice, tobacco, tomato, wheat, barley, sugarcane were selected for toxins of Fusarium, Helminthosporium, Alternaria and Phytophthora. In vitro selection for disease resistance presents an excellent opportunity to assess the direct application of tissue culture to crop improvement.
Cell Line Selection for Draught and Salinity Resistance:
ADVERTISEMENTS:
The need for plants with increase tolerance to environmental stress is well known. At least 25% of currently cultivated land suffers from salinity, mainly from NaCl. An additional 25% from acidity, most often caused by alluminium ions, and 40-60% from draught. Establishment of totipotency in plants resulted in regeneration from selected material.
Cells are grown on media containing NaCl or polyethylene glycol (PEG 4000 or 6000) for several passages, then grown on medium without selection pressure (PEG, NaCl) and again on the media containing selection pressure.
Cells survive on such conditions are supposed to be resistant to the stresses. These cultures are regenerated and again tested for resistance against draught and salt. Such plants have been developed in barley, potato, rice and tobacco.
Cell Line Selection for Nutritional Quality:
Selection of cells for nutritional quality includes improved levels of total amino acids, individual amino acid like leusine, methionine, tryptophan, threonine, and vitamins. Such cell lines were developed by mutations and use of amino acid analogue. These analogues block the pathway preventing metabolism of the amino acid. This results in increased amino acid accumulation.
Various types of mutants for amino acids are known in Arabidopsis, tobacco and datura. This line did not produced many improved crop plants therefore other methods like gene transfer are employed, resulting in production of golden rice, improved for vitamin A.
Selection Parameters:
Clones can be selected on the basis of visual (morphological) characters, growth characteristics and physiological parameters (protein banding, secondary metabolite production). If colonies are colored (pigmentation: anthocyanins, chlorophyll: presence-absence) or their fluorescence properties evident under ultraviolet light (secondary products), it is much easier to select producer colonies.
This procedure has been effectively used in selection of high-ajmalicine producing cells of Catharanthus roseus and pigmented anthocyanin and shikonin producing cells in Vitis vinifera and Lithospermum erythrorhizon cell cultures, respectively.
Once the clone is grown in size, sufficient to divide and subculture, part of the clone is used for subculture while the other part is retained for qualitative/quantitative secondary compound determination.
Once all clones are quantified for their useful metabolite contents, then high secondary metabolite-containing clones are selected and the poorly productive may be discarded or screened for new product/s. Any of the following criteria is used to select the clones depending on the nature of secondary metabolites.
Visible markers:
Coloured pigments and compounds fluorescent under UV light may be used as visible markers for selection of clones. Invisible compounds as markers: for such compounds a quick analytic system sensitive enough to detect minor quantities of secondary metabolites is required to evaluate the clones.
These are:
(i) Chemical tests like Dragendorff’s reagent for alkaloids or Libermann-Buchard test for steroids etc.
(ii) Thin layer chromatography of crude samples and use of specific reagent as used for cultures of that particular species.
More sensitive methods such as GLC, HPLC, RIA may also be used which requires partial purification of samples before analysis is carried out.