ADVERTISEMENTS:
Read this article to learn about the isolation of plant protoplasts by enzymatic method, plant protoplast culture, somatic hybridization and fusion techniques used in plant protoplasts.
Isolation of Plant Protoplasts by Enzymatic Method:
Protoplasts are isolated by two methods, mechanical and enzymatic. Mechanical isolation method of protoplasts is no more practically used. It is only a historical method. This involves cutting of plasmolysed cells (in fact cell walls) with a sharp blade to release protoplasts.
This involves four steps:
ADVERTISEMENTS:
(a) Sterilization of leaves
(b) Peeling off the lower epidermis
(c) Incubation in enzyme solution and
(d) Isolation and cleaning of the protoplasts.
ADVERTISEMENTS:
Healthy leaves are obtained from green house grown plants and leaves are sterilized as described earlier (sterilization of explants). After sterilization lower epidermis is removed with the help of fine forceps and the stripped leaves are cut into small pieces.
Flaccid leaves facilitate peeling. In vitro grown cultures are aseptic material and therefore, used directly for the isolation of protoplasts. Actively growing cultures in the exponential phase are good material for the isolation of protoplasts.
Usually one gm. material is used o obtain protoplast and conditions are optimized empirically by changing enzyme concentration and composition, concentration of osmoticum, duration of incubation and types of tissues.
Enzymes:
Different enzyme preparations are available in the market but the idea is to combine one middle lamella dissolving and one cell wall digesting enzymes in proper composition to achieve maximum protoplasts release from one gm. material.
Following enzymes are used:
1. Macerozymes R-10
2. Cellulase – Onozuka R-10
3. Hemicellulose
ADVERTISEMENTS:
4. Pectinase
5. Drieselase
A combination of these enzymes in a concentration of 0.5-2% is used. In many cases only macerozyme and cellulase are sufficient to obtain protoplasts in significant number. The enzyme solution (pH 5.5) is prepared in 10-15% sorbitol or mannitol containing small amount of CaCl2 (7 mM) for membrane stability.
This solution is sterilized through a membrane filter (cold sterilization) and leaf or callus tissues are placed in it. Petri-plates containing tissue and enzyme mixture are sealed with parafilm and incubated for 4-12 hours (sometimes 0.5 to 20 hrs.) on a rocking shaker at 24-26 °C.
ADVERTISEMENTS:
After incubation, solution is filtered through a wire or nylon mesh (50-100 µm) to remove debris (undigested cells, tissues, broken cells etc.), transferred into screw capped small centrifuge tubes (sterilized), and centrifuged at 100 g. The protoplasts formed a pellet while the debris in the supernatant is carefully removed.
Fresh sterilized sorbitol solution (no enzyme) is added to tube and centrifuged. By repeating the process 2 to 3 times, protoplasts are cleaned (debris is removed). If 20% sucrose solution is used, protoplasts will float and debris will settle during centrifugation at 200g for 1 min.
Floating protoplasts are carefully removed with the help of sterilized pipette and bulked together for further use. Protoplasts are counted by haemocytometer and then diluted to proper strength (number per ml) in the culture medium containing osmoticum (Fig. 30.1).
Protoplasts Culture:
Isolated protoplasts require somatic protection in the culture medium until they generate a strong wall (Fig. 30.2). Osmolarity in the medium is adjusted to the same level as in the enzyme and washing solution. Prolonged culture at high osmotic pressure can result in browning of the cultures and inhibition of callus growth. The osmolarity of the medium is gradually decreased with cell wall formation and cell divisions. Abrupt decrease in osmolarity may cause bursting of protoplasts and cells and also affects the growth of cells.
Generally the basic constituents in protoplast culture medium are similar to those used for cell cultures viz. MS or B5 medium. Some modifications can have beneficial effects on protoplasts survival and colony formation. Usually carbon, nitrogen, vitamins and plant growth regulators are suitably modified. Successful culture of plant protoplasts often depends on the appropriate medium volume per cell ratio (density). The optimal protoplasts density usually has to be experimentally determined. The usual density range from 0.5 to 2 x 105 protoplasts per ml of culture medium.
Protoplasts Fusion and Somatic Hybridization:
In nature, gametic fusion forms zygot with a new combination of genes. Diploid nature is restored by haploid gametic fusion. Sexual crossing is one of the major sources of genetic variability. Incompatibility at various levels, before fertilization and after fertilization, restricts recombination between distantly related species and species specific characters are maintained.
A limited success has been achieved in obtaining inter-specific and inter-generic hybrids by conventional breeding programmes. These hybrids are useful in further breeding programmes and in the understanding of cytological and genetical behaviour of chromosomes in these hybrids.
ADVERTISEMENTS:
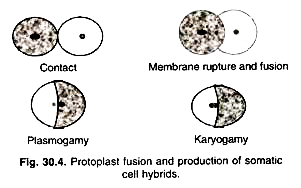
However, it is not possible to obtain hybrids by conventional method in all the desired plant materials. Protoplasts fusion provides a novel approach overcoming sexual incompatibility and obtained somatic hybrids. Fusion of somatic cells (2n) and production of hybrids is known as somatic cell fusion or somatic hybridization.
Plant membranes are flexible, asymmetric structure of membrane proteins integrated into phospholipid molecules. The fluid mosaic model with lipid bilayer is the established structure of intact membranes. The process of protoplasts fusion requires the direct contact between membrane surfaces and integration of membranes of two different cells.
The adhesion of protoplasts depends on surface charge. According to electro-phoretic studies outer membrane surface is negatively charged. This change varies from -10 mV to -50 mV and can significantly be reduced by Ca2+.
Due to these fusion properties of the membranes, protoplast fusion has been achieved between dicot and dicot, dicot and monocots and plant and insect cells (Drosophila), amphibian cells and mammalian cells. Protoplast fusion between haploid (gametic) and diploid (somatic) cells has been achieved which clearly demonstrate that fusion is independent of cell type. Protoplasts of both types are mixed in an equal proportion attaining a density of 5 x 104 to 2 x 105 protoplasts per ml. Protoplasts density is usually determined by haemocytometer.
There are several chemical and physical agents who enhance fusion between protoplasts, known as fusogen or fusion agent, e.g., sodium nitrate, polyethylene glycol of different molecular weight (PEG 1500, PEG 4000, and PEG 6000), dextran sulphate and gelatin etc. High calcium ion concentration and pH of the incubation mixture have profound effect on fusion percentage. Fusion is stimulated under electric charge as membranes are negatively charged.
Isolated protoplasts are suspended in an aggregation mixture (5.5% sodium nitrate /20-40% PEG in 10% sucrose solution), kept at 35 °C for 5 minutes and then centrifuged at 200 g for 5 min to obtain dense pellet. The tube with pellet of protoplasts is once again transferred to a water bath maintained at 30 °C for 30 minutes during which most of the protoplasts undergo fusion. The protoplasts are gently washed and transferred to liquid medium or plated. The fusion and fusion products are observed under inverted microscope.
Fusion Techniques Used in Plant Protoplasts:
While several methods were proposed to induce protoplasts fusion, earliest success was achieved using sodium nitrate (Carlson et al, 1972). Plant protoplasts have uniformly negatively charged membranes. Divalent cations, especially Ca2+, modify the electrophoretic mobitility of protoplasts. Aggregation mediated by neutralization of plasma lemma negative charges by 50 mM Ca2+ mixed with 0.4 M mannitol in a solution of high pH (10.5) has led to the genuine cytoplasmic fusions of tobacco protoplasts and Petunia protoplasts.
ADVERTISEMENTS:
The other fusogens are as follows.
1. Peg:
Various fusogenic agents have been used for getting protoplasts fusion. The discovery of the powerful aggregation effect of polyethylene glycol (Kao and Michayluck, 1974) provided the awaited tool. A few minutes after being immersed in a 20-40% (w/v) solution of PEG (1500 to 6000 mw) virtually all protoplasts exhibit adhesion.
However, actual fusion occurs upon dilution of PEG. A combination of PEG induced adhesion followed by elution with PEG high Ca2+, high pH was even more reliable. Actual role of PEG in the process of membrane fusion remains yet to be clearly determined.
Addition of dimethyl sulphoxide (DMSO) with PEG has stimulatory effect on protoplast fusion. It has been suggested that bridge formation mediated by Ca2+ and PEG through its slightly negative polarity might be the cause of PEG induced fusion of protoplasts.
2. Dextran:
Similarly fusogenic properties of dextran and of polyvinyl alcohol suggest that weak non- ionic surfactant properties be involved. It seems perturbation of negative charges of the protoplast plasma lemma seems essential. This is illustrated by fusion induced by a positively charged phospholipid and above all electric field induced fusion.
3. Electro Fusion:
ADVERTISEMENTS:
In electro fusion method of Zimmerman (1982) protoplasts are brought in close contact by a non-uniform alternating field between two electrodes, then fusion is initiated by field pulse of high voltage (500 V – 3 KV, cm-1) for a very short time (10-100 micro seconds). This is the most widely used apparatus (Fig. 30.3) and method in the studies on membrane fusion and for the production of somatic hybrid colonies or plants.
Electro-fusion is a biophysical process that involves two steps. In the first step the protoplasts are exposed to alternating electric field (0.5-1.5 MHz) that generates dipoles through di-electrophoresis. In this non-uniform field, the protoplasts acts as dipoles, move in the direction of the increasing field as a function of the field strength, cell size and the mobility of charge within the protoplast and in the surrounding solution.
As a result of mutual di-electrophoresis in non-uniform as well as uniform field a protoplasts clump, and bind together to form the chain. The second step for electro fusion is the application of one or more short (10-100 µs) direct current (DC, 103 KV/cm) pulses that cause reversible membrane breakdown. This results in pore formation in the membranes touching to each other. This way contacting membranes can be fused and this fusion opens the way for hybrid cell formation. Generally several cycles of AC and DC current are given to increase the fusion frequency.
The fusion chamber is a small apparatus having two parallel running platinum wires for passing the current (Fig. 30.3). Protoplasts suspension is placed in this micro-chamber having platinum electrodes (wires). This can be placed on inverted microscope and fusion process can be observed simultaneously (Fig. 30.4). PEG fusion efficiency is given as the best 5-30% hetero-specific fusion products under optimal conditions. The electric fusion method appears very promising, as its efficiency is very high.