ADVERTISEMENTS:
Here is a list of seventeen major diseases that are found in maize: 1. Smut Diseases 2. Virus Diseases 3. Downy Mildews Disease 4. Brown Spot Disease 5. Stalk Rot Disease 6. Pre-Flowering Stalk Rot Disease 7. Pythium Stalk Rot Disease 8. Post-Flowering Stalk Rot Disease 9. Charcoal Rot Disease 10. Black Bundle Disease 11. Cob Rots Disease 12. Fusarium Ear Rot Disease 13. Aspergillus Ear Rot Disease and Others.
Diseases found in Maize:
- Smut Diseases
- Virus Diseases
- Downy Mildews Disease
- Brown Spot Disease
- Stalk Rot Disease
- Pre-Flowering Stalk Rot Disease
- Pythium Stalk Rot Disease
- Post-Flowering Stalk Rot Disease
- Charcoal Rot Disease
- Black Bundle Disease
- Cob Rots Disease
- Fusarium Ear Rot Disease
- Aspergillus Ear Rot Disease
- Foliar Blight Disease
- Banded Leaf and Sheath Blight (BLSB) Disease
- Brown Stripe Downy Mildew (BSDM) Disease
- Polysora Rust Disease
1. Smut Diseases:
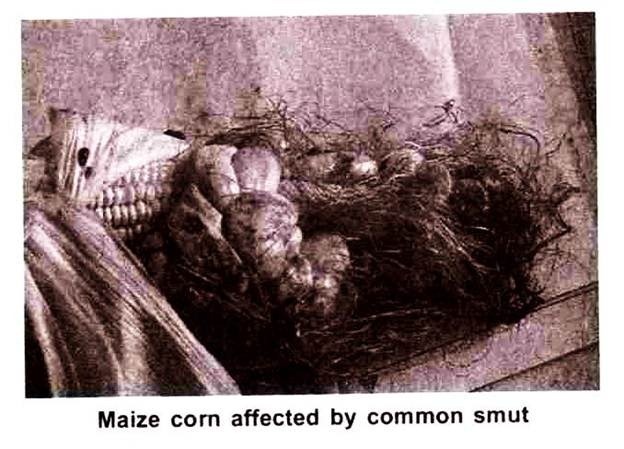
i. Common Smut (Ustilago maydis (DC) Cda:
ADVERTISEMENTS:
The characteristic galls are formed on the infected tissues. The gall may appear on the stem, leaves, axillary’s buds and parts of the male flower. When formed on the cob they cause extensive damage. The developing mycelium between the thin walled cells induces hypertrophy.
The galls are light coloured in the beginning and later on become dark. As the galls are enlarging they appear light coloured or almost white.
The individual flowers may be infected on tassel and even through individual flower parts may be transformed into galls. The light, shiny membrane ruptures and dry black spores masses are exposed. The black powdery masses are the chlamydospores of the fungus. Stem galls result in loss in yield and bending of the stalk.
ii. Head Smut [Sphacelothica reilina (Kunh.) Clinton]:
ADVERTISEMENTS:
This attacks maize, sorghum and other grasses and is moderately destructive disease in the sub-temperate, intermediate zone of hilly areas of Rajouri and Poonch districts of J&K. It has occurred in severe form on Pioneer H-3054 and caused 25-35 per cent yield losses during Kharif-2005 in Manialdara region of Rajouri district.
It appears with the formation of tassels and ears where partial or complete transformation of the inflorescence into sori may be observed. The tassel is partly or wholly converted into smut spores. Galls are first covered with a membrane that soon breaks open to expose a powdery mass and the vascular bundle of the host.
If only the parts of the tassels and ears are converted into galls, the floral bracts turn into leafy structures. Ears frequently abort and are replaced by leafy buds. It is externally seed borne and soil borne. Galls on the ear usually destroy it to a large extent while large galls above the ear cause much greater reduction in yield than do galls below the ear.
Losses from corn smut are highly variable from one location to another and may range from a trace upto 35 per cent or more in localized areas. The spore retain viability for two years. Although, the disease may be externally seed-borne, the major source of infection is soil-borne inoculums. Only young plants are susceptible. Low temperature is favourable for infection.
Control Measures:
i. Field should be cleaned off the trash after harvest. Since only few plants are affected in a field it is possible to locate and destroy the infected ears before they shed the ears.
ii. Follow up crop rotation.
iii. Seed treatment with captan or thiram at the rate 3 g/kg seed.
ADVERTISEMENTS:
iv. Grow resistant/moderately resistant var. like KH-517, KH-612, GS-2 etc.
2. Virus Diseases:
Viruses are the most mysterious disease agents affecting both plants and animals, and hence the limited amount of studies and where there is the least information on their incidence and economic effects.
The lack of information is more acute in the developing countries, where there is a lack of trained personnel and appropriate equipment, and mostly to the concept that when these viral diseases are present, nothing can be done to control them.
ADVERTISEMENTS:
The problem with such mentality, the incidence of these diseases continues to increase, especially under the present actively changing circumstances in agriculture when new germplasm is almost freely moving throughout all environments, agronomic practices are changing, and there is a conspicuous expansion of area where maize is being grown due to an increased demand.
These changes in technology are bringing along an increase in problems, specially insects and diseases, that were not previously considered problems of any significance.
Three viruses occurs on maize in India. These are maize mosaic virus I, maize mosaic (a strain of sugarcane mosaic virus) and vein enotion. The information is required to both pathologists and breeders to understand the complexity of the viral pathotypes, both in their genome and their evolution, to be considered when selecting for stable resistance to their pathogens.
Mosaic Virus (Mosaic Virus Transmitted by Leafhopper):
ADVERTISEMENTS:
Small white fleck may occur on one side of the midrib near the base of the young leaf and is usually associated with whitening of veins. The specks elongated to form fine discontinuous stripes. Sometimes the stripes coalesce and form yellow bands of the leaves.
Stripes may also be formed on sheaths, husks, and the stalks. The plants are pale green, stunted and show mosaic mottling on the leaves. Cobs are not filled well.
Control:
Grow resistant hybrids.
ADVERTISEMENTS:
Maize Dwarf Mosaic Virus (Sugarcane Mosaic Virus, Transmitted by Aphids, Myzus persicae):
Early infested plants produce long chlorotic streaks along the veins at the base of the leaf lamina. With the growing age of plant, leaves in the whorl become chlorotic and in turn the chlorotic areas in leaves turn red or purple.
Control:
Sow resistant varieties.
Maize Mosaic:
This has been found to be prevalent in the several states and the incidence ranges from 2.2 to 10.6 per cent. Resistance of a high order even immunity (CM 103 and CM 104) has been identified and improved released cultivars have not been reported to suffer much from virus diseases.
ADVERTISEMENTS:
3. Downy Mildews Disease:
ADVERTISEMENTS:
Ten downy mildew (DM) diseases are identified on maize. Of these, seven are caused by Peronoslerospora species, two by Sclerospora and one by Euclerospora. All the Peronoslerospora incited diseases are of old world origin, three of which namely, P. sorghi, P. sacchari, and P. phillipinensis are of common occurrence in India.
i. Leaf Rust : Puccina sorghi:
Rust pustules appear on the above ground plant parts especially on leaves. Round to elongated uredo-pustules are on both the surfaces of the leaf. They may coalesce and thereby produce scorching or drying of the leaves.
Later on these pustules turn black due to formation of teleutospores. This disease is air born. The secondary infection take place by means of uredopustules which are wind born disseminated to the neighboring plants. The major approach adopted has been the utilization of host resistance. Both hybrids and V composites possessing resistance have been released.
ii. Sugarcane Downy Mildew (Sclerosspora sacchari Miyake):
ADVERTISEMENTS:
The characteristic symptom is the development of long, rather broad chlorotic stripes along almost entire length of the leaves. The colour of the stripe is whitish in early stage which changes to dark brownish yellow in very late stages. The growth of the fungus can be seen on both the surfaces of the stripes. The affected plants may be malformed with undeveloped tussles and ears.
The cobs are poorly filled. Source of primary inoculum includes collateral cultivated and wild hosts, infected volunteer plants, kitchen gardens and certainly oospores, where formed. Infection usually begins with a few plants in early sown fields. Subsequently adjacent planted fields can be severely damaged.
The Peronoslerospora spp are seed borne in nature. However, they do not appear to present a problem. Oospores have not been implicated as being carried out on kernels surface. They could be shaken off or killed chemically. Internal mycelium is inactivated by drying seed to 20 per cent or less moisture and storing for about 3 weeks.
iii. Philippines Downy Mildew (Sclerospora philippinensis):
The symptoms are very old similar to that of sugarcane downy mildew except for intensity of colour of stripes. The leaf infection results in long, chlorotic stripes with downy fungal growth. Affected plants produce malformed tassels or aborted ears which may appear at any time of silking, but the plants affected early are stunted and often die.
The downy mildew fungi are mostly on alternate hosts and perenate through oospores which falls down in the soil and secondary infection takes place by means of conidia which are wind disseminated.
iv. Peronosclerospora Downy Mildew:
Greatest loss results from systemic infection. This results in death or bareness. Losses from India and several SE Asian countries have been reported to be as high as 40-60 per cent. In India, significant loss in maize yield is usually localized to late planted areas. South India especially in TN and Karnataka have been reported epidemic at various times.
4.
Brown Spot Disease:
This disease mainly occurs in sub-tropical and intermediate areas. It is distributed in the entire Himalayan area as also in states of Rajasthan and Karnataka. Resistance has been identified on the basis of natural incidence only and methods to induce artificial disease development in the field through resisting sporangia are to be worked out.
A study conducted at Rajasthan, indicates that the yield loss in hybrid Ganga 5 was 27.0, while in the local open-pollinated variety it was Malan 24.5 per cent.
5.
Stalk Rot Disease:
Stalk rots are the most serious and widespread group of the diseases in maize. “The term “stalk rot” is often used to include stalk breakage, stalk lodging, premature death of plants and occasionally root lodging. In true sense, it is decay of the internal pith tissues of the stalk. Typically, the first sign of stalk rot is plant wilting. In initial stage, leaves become gray, ears drop and outer rind of lower stalk may turn brown.
When outer stalk tissue is brown, pith tissue in the lowest internodes is rotted and pulled away from the rind, which may result in lodging. Plants are weak and those with rotted stalks always have rotted roots. There nature is often complex as a number of fungi, nematodes and sometimes bacteria are involved in causation of the disease(s).
This group of disease is broadly divisible into two categories, viz., pre-flowering and post-flowering types. Of the 12 diseases recorded so far, Pythium stalk rot (P. aphanidermatum) and bacterial stalk rot (Erwinia chrysanthemi pv. zeae) belong to pre-flowering type, while others such as late wilt, charcoal rot, Fusarium wilt and stalk rot, Acremonium stalk rot, Botryodiplodia stalk rot belong to the post-flowering type.
Losses due to stalk rot may occur in the following three ways:
i. Yield loss may result from premature plant death, thus stopping normal grain fill. Total grain weight on stalk rot affected plants is less than the weight on healthy plant.
ii. Another component of yield loss is that plants with stalk rot may lodge and not be harvested with mechanical equipment. Harvest is slowed if stalk rot is severe and losses also occur due to time loss during harvest.
iii. Losses also occur with ear rot as a results of the ear on lodged plant coming in contact with soil. This results in reduced grain quality and potential dockage when the grain is marketed. Because losses due to stalk rot may occur in several ways, yield loss estimates are difficult to obtain.
Technique used to estimate losses are:
i. Yield of hybrids with practically nil natural stalk rot incidence have been compared to yields in years when stalk rot is severe. These types of studies are confounded by year-to-year in average yield.
ii. Another technique used is comparing inoculated vs. uninoculated plots. These studies, however, are confounded by natural infection in uninoculated plots, and by the fact that inoculation does not exactly duplicate the natural stalk rot condition.
iii. In paired plant technique, grain yields of adjacent diseased and healthy plants are compared. In this, the problem in diseased plants have much less kernels than on healthy plants. The final problem with yield loss studies is that the losses are estimated on the basis of hand-harvested yield and do not take into account those losses due to lodging or ear rots.
6.
Pre-Flowering Stalk Rot Disease:
Erwinia Stalk Rot (Erwinia chrysanthemi pv. zeae Victoria, Arboleda and Munoj):
It is one of the most important disease of maize in tropical countries. At first, the upper leaves show signs of wilting. The symptom appear at the lower node and may remain confined at one or two internodes only. The inner tissues also get infected in severe cases.
The infected tissues are at first soft but later on they turn into dry mass of shredded fibers. The plants may topple down at this stage. In some cases the infection (light to dark brown rotting) may spread rapidly throughout the leaf sheath and cause withering of leaf sheath and leaf under favourable environmental conditions.
The stalks and ears may also be affected which emit an offensive odour. High disease incidence is linked with irrigation by sewage water, it is particularly favoured by high temperature (28°C and above) and the high ambient moisture which commonly prevails in the most of the maize growing areas 3 to 4 week after sowing.
The disease can be minimized, using indigenously formulated compound bleaching powder containing 33 per cent chlorine at the rate 10 kg /ha as soil drench at pre-flowering in standing crop. Avoiding of water logging and proper drainage also helps in reducing disease incidence.
Planting the crop on ridges rather than in flat soil is recommended. Spraying mixture solution containing 50 g Agrimycin and 2 kg of ceresan wet/ha will also control the disease.
7.
Pythium Stalk Rot Disease [P. aphanidermatum (Eds.) Fitzp.]:
The disease is common during rainy season under hot and high soil moisture conditions. The most diagnostic feature of this disease is that the rot is contained mostly to a single basal internode leading to destruction of pith parenchyma and consequent weakening to the stalk. The affected plants topple over but do not die for upto 2 weeks after attack.
The general symptoms of the disease are poor emergence or patchy growth of seedlings or the seedlings become pale yellow or dry up just after germination. On pulling out the affected plants, rotting of the seeds may be clearly seen. Stalk rot is first recognized when the plants first lodge but do not break over. Stalks often rot at the first, internode above the soil level and become soft and brown.
This disease has been reported in Kliarif season in areas having temperature ranging 30-35°C and RH (relative humidity) 80-100 per cent. When RH drop 50 per cent or below, no rot develop in the stalks. This explain why high disease incidence occurs when the field is water logged, low lying or poorly drained, plant age (pre-flowering stage) and high plant population (< 60,000 /ha).
The tooth pick method as described for post flowering stalk rot development can be used to inoculate plants at knee high stage (30-35 days) for screening genotypes, Payak (1971), Payak and Sharma (1985).
8.
Post-Flowering Stalk Rot Disease:
Fusarium Stalk Rot [Fusarium moniliforme Sheld. = Gibberella moniliforme (Sheld.) Wineland]:
The disease appears both on young and adult plants of maize. In young seedlings the plants dry up in the early stage of the growth. In the adult plants the symptoms are not visible till maturity. At this stage drying of top leaves from tips begin showing water scarcity. Ultimately the entire plant dries up.
The cob formation either does not take place or the grain formation is partial or wholly affected. The characteristic reddening of the internal tissues are discernible when affected plants are longitudinally split open. In some cases the red streak may also be observed. The pith becomes hollow and white to pale pink mycelial growth may also seen in the cavity thus formed in advance stage of disease development.
Disease Management:
i. Sow the resistant varieties like GS-2 and Ganga-101 which are comparatively resistant.
ii. Follow 2-3 years crop rotation.
9.
Charcoal Rot Disease [Macrophomina phaseolina (Mubl.) Ashby]
This is a common disease in warm and dry areas. A characteristic sign of disease is the presence of numerous, minute, black sclerotia, particularly on the vascular bundles and inside the rind of the stalk. This may cause the stalk to appear gray-black.
The disease symptoms appear only near maturity. Water soaked, brown lesions appear on roots which turn brown later on. The disease is favoured by high temperature i.e. 30-42°C and low soil moisture. The pathogen over winters as sclerotia and may penetrate roots and lower stems during growing seaspn.
integrated Disease Management in Maize
Avoid water stress after flowering of the plant.
10.
Black Bundle Disease (Cephalosporium acremonium Corda):
The characteristic symptoms is the blackening of the vascular bundles as black dots on the cut ends of the stalk. Other important symptoms of this disease are a reddening or purpling of the leaves and stalks, lesions on basal portion of the stalk, multiple ear formation at node and excessive tillering. In severe cases, leaves dry and plants may wilt. Ears may rot or even the ears may not be formed.
Seed treatment with carbendazim or benlate at the rate 2 g/kg seed.
11. Cob Rots Disease:
The safety and integrity of the food supply are the paramount importance and are among the drivers of safe grain storage. In present scenario, the trade among the developing countries is expanding; hence quality of the produce is becoming a major concern.
Demand for maize in both national and international market is high. Its uses are continuously increasing globally as important livestock, poultry feed and by industrial sectors.
On the other hand the loss of grains is increasing with the increase of food production due to poor and improper storage as well as the pre harvest fungi associated with maize. These fungi are responsible for microbial spoilage of the grins and production of mycotoxins in pre- and post harvest stages.
Symptoms and Epidemiology:
Fungi associated with the cob rots are— Aspergillus flavus, Fusarium species, Gibberella zeae, Penicillium etc. These field fungi infect the cobs either immediately or before harvest. The presence of mycotoxin traditionally regarded as indication of poor storage condition, but they may present in grains before coming to storage.
12.
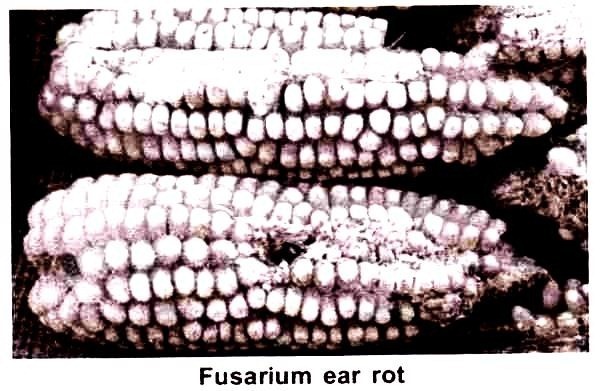
Fusarium Ear Rot Disease:
Fusarium ear rot caused by F. moniliforme symptom of this disease is pink to reddish brown discoloration on the kernels and later on it spread on whole ear. As the disease progress, infected kernel becomes covered with a powdery/cottony pink mild growth.
Kernels infected late in the season develop whitish streaks on the grains. Kernels become infected in several ways. The most common pathways is infection via silk channel. Air born spores present on residues can land on corn silks when it turns dark brown. Green silks are relatively resistant.
Infection follows some form of injury, bird damage, feeding of corn borers. Disease development and spread one favoured by dry warm weather. Under certain conditions that are stressful for maize plant as the fungus becomes pathogenic and causes disease. Stalk rot, ear rot, kernel rot can occur in infected tissue although many times infected tissue have no symptoms.
13.
Aspergillus Ear Rot Disease:
This rot caused by Aspergillus spp. and infection often follows drought stress and damage done by corn, earworms and corn borer and other insects. A tan sooty black, greenish or greenish yellow mold grows on and between kernels. The damage is most common at or near the tip of the ear; silk infection is favoured by high night and day temperature.
Some times the susceptible maize genotypes got infected with Aspergillus sp. at the time of silking. The infection goes through silk to the maize seed/grains but remained latent and cause decay at the time of maturity in the form of cob rot/ear rot, sometimes the grain remain infected but does not show any symptoms even at the time of maturity.
Corn planted and harvested late and grown under nitrogen stress more commonly contains aflatoxins prior to harvest than corn grown under good management practices and supplied with adequate nitrogen.
Maize grown in cooler areas usually contains low amount of mycotoxin.
14. Foliar Blight Disease:
Of the many foliar blight diseases occurring in India, three may be considered important based on geographical distribution and yield loss potential.
Northern leaf blight (Heirninthosporium turcicum = Exserohilum turcicum) is a major problem in the region with cooler environment like J&K, H.P. etc., under conditions favourable for disease development, susceptible materials suffer heavy blightening resulting in premature drying of leaves and lightweight kernels.
It is a major disease in the mountainous areas and in the deep hillside areas of Rajouri and Poonch districts, especially in the cool and shady environment of intermediate zone of J&K. The highest disease intensity i.e. 66 per cent was recorded in intermediate zone of Jammu region.
The foliar blight disease complex in cool conditions favoured for turcicum leaf blight. At the Regional Agricultural Research Station, Rajouri and MBRSS, Poonch, it was moderate to high. The natural disease pressure every winter cycle allowed for the selection of disease resistant germplasm.
It is important that, where a disease outbreak is feared, spraying should be undertaken at an early stage of crop growth, i.e. when it is not more than 30 days old (knee-high stage).
For large scale high volume sprays 1.8-2.6 kg of fungicide in 900-1200 litre of water/ha is recommended, if the plant population is around 56,000/ha. Control of foliar blight disease can be achieved using host resistance which has been quite successful, chemical control is also feasible, it becoming profitable on high volume materials like popcorn, sweet corn, baby corn or seed crop.
15. Banded Leaf and Sheath Blight (BLSB) Disease:
It was first reported from Sri Lanka. In India, it was first recorded by Ullstrup in the Tarai areas of U.R. Now it is present in J&K, H.P., U..P, M.P., Haryana, Punjab, Rajasthan, W.B., Meghalaya and Assam. This disease has been considered as one of the major diseases of maize.
Grain losses have been estimated in the range of 23.9-31.9 per cent in ten cultivars. Singh and Sharma (1976) estimated 40.5 per cent loss in grain yield with 71 per cent disease index.
The magnitude of grain loss may reach as high as 100 per cent if the ear rot phase of the disease predominates. The disease appears on plants at pre- flowering stage (40 to 50-day old plant) and within a period of 15 to 20 days spreads under favourable conditions from the lower most sheath to the ear shoot.
Rhizoctonia solani Kunh is morphologically characterized by features like pale to brown colour of mycelium, branching near the basal septum in young growing hyphal, presence of a construction and formation of a septum in branch near the point of origin, absence of clamp connections, sclerotia of un-differentiated texture, young multinucleate hyphal cells with a prominent septal pore apparatus and rapid growth rate.
The primary source of inoculums are sclerotia in the soil and grass hosts that grow in the vicinity of maize crop. Secondary spread of disease is by contact of infected leaves with parts of adjoining healthy plants. Secondary spread by basidiospores has not been observed in maize crop.
The optimum temperature for in vitro growth of the pathogens is 30°C and the highest level of disease is induced when RH is in the range of 90-100 per cent. At RH of 70 per cent lower, the disease development is negligible or absent.
It is caused by incited by Thanatephorus sasakii (Shirai) Tu & Kimbro; anamorph Rhizoctonia solani f.sp. sasakii Exner, has become increasingly severe and economically important disease of maize during last two decades or so. For the first time was reported from Srilanka under the name ‘Sclerotial’ disease.
Though it was considered a disease of minor importance till it appeared in an epidemic form in the foot hills region of Himalayas especially in the district of Mandi in Himachal Pradesh.
In India, in early sixties the disease was little more than a curiosity and a disease of minor importance in the western and central Himalayan foothill region. However, it became increasingly severe and assumed epidemic proportions in the next two decades. Presently, the disease is considered as a major disease not only in India but also in several countries of Tropical Asia wherever maize is grown.
On maize the disease was first recorded from Sri Lanka under the name ‘sclerotial disease’. In recent years the disease outbreaks have occurred in more countries and have assumed epidemic dimensions. In India, the disease was first recorded from Tarai region of Uttar Pradesh in 1960.
Now it has become increasingly severe and assumed epidemic proportions in the next two decades. Presently, the disease is considered as a major disease in India wherever maize is grown in warm and humid conditions.
It is known to be present in severe intensity in the states of Himachal Pradesh, Uttar Pradesh, Uttaranchal, Haryana. Punjab, Madhya Pradesh, Rajasthan, Bihar, West Bengal, Sikkim, Meghalaya, Assam and Odisha.
The disease was earlier reported as a minor disease on maize. The importance of the disease was only realized in early 1970s when an epidemic occurred in warm and humid foot hills area, particularly in the Mandi district of Himachal Pradesh.
The disease causes direct losses, resulting in premature death, stalk breakage and ear rot indirect losses by reducing the grain yield. In India, losses in grain yield have been estimated in the range of 23.9 to 31.9%.
Singh and Sharma (1979) estimated 40.5% loss in grain yield with 71% disease index. However, the magnitude of grain loss may reach as high as 100% if the ear rot phase of the disease predominates. Payak and Sharma (1985) have reported that annually at least 1% of the total grain yield is reduced by BLSB in India.
Losses to the extent of 11-40 per cent were reported while evaluating 10 different varieties of maize. Losses in grain yield showed a high positive correlation with premature death of plants and disease index.
The disease caused drastic reduction in grain yield-to the tune of 97 per cent and exhibited a direct correlation with other yield parameters. Disease incidence varying from 2 to 80 per cent was observed affecting maize crop severely in Korea during previous two crop seasons.
The disease generally appears at pre-flowering stage in 40-50 day old plants. The symptoms manifest on leaves, sheaths, stalk and ear. As the disease is soil borne it starts from the lowest leaf sheath or on leaves that are in contact with soil and travels up to the ear.
The symptoms are more common on the leaf sheaths than on the laminate. The disease lesions are characterised by the presence of alternate bleached areas or zones that are initially water soaked and narrow, purple brown bands oriented perpendicular to long axis of leaves or leaf sheaths.
The hyphal masses on ears produce a caking effect and cement the husk leaves as well as styles (silk fibers) together. The mycelium develops above and between kernel rows. Sclerotia are found to be produced not only on sheaths, leaves and husk leaves but were also observed for the first time on glumes, in cupules, under the pericarp in caryopsis and silk fibres.
16. Brown Stripe Downy Mildew (BSDM) Disease:
It has been most severe in U.P., H.P., southern Rajasthan, Punjab, hilly part of W.B., J&K especially in areas that receive 100-200 cm of rains. A yield loss of 63 per cent was recorded in the tarai areas of U.P. As the leaves are infected by the pathogen, it drastically reduces the total photosynthetic area of the diseased plants, causing reduction in grain yield.
The yield losses depend on susceptibility of the host, spread of the disease and environmental conditions prevailing in the growing season. This is more destructive disease in the intermediate zone of Jammu region. Disease symptoms have been observed only on leaves. In the initial stages, the lesions start developing on lower leaves as narrow chlorotic or yellowish stripes, 3-7 mm wide but variable in length.
The stripes extend in parallel fashion, have well defined margins and are delimited by veins. The presence of downy whitish to creamy growth usually on the ventral surface of the infected leaves corresponding to- stripes is the most characteristic symptom. In cloudy weather, the growth is profuse and can be seen. Later, these chlorotic stripes turn brown and give a burnt appearance to the leaves.
The Pathogen is an obligate parasite and cannot be grown in artificial media. It is mainly soil borne. Its seed borne nature is controversial. Some workers reported this pathogen as seed-borne but it was found later that S. rayssiae var zeae is not seed borne because no disease symptoms were observed in spite of providing ideal conditions to the pathogen grown from infected seeds.
There seems to be no controversy about soil borne of the disease. Oospores remain viable for at least three years and this viable oospores material constitutes the main source of inoculums. Primary infection invariably occurs on lower proximate to the ground level. Once the primary infection becomes established, its spread is possible through the agency of sporangia.
A definite relationship of disease development with rainfall pattern in different region ns has been mapped. In areas where rainfall varies from 40-60 cm, disease was observed from trace to low, in 60 to 100 cm rainfall areas it was from low to moderate, but in the areas having more than 100 cm rainfall, maximum disease ratings have been made.
However, in areas of low rainfall, disease may be severe only when continuous spell of shower and cloudiness for a few days accompanied by about 25°C temperature are prevalent. The severity of the disease is influenced by temperature and moisture. Sporangia are produced at low temperature (20-22°C), while oospores are formed at high temperature. Soil temperature of 28-32°C favour disease development.
Sporangial production and infection require a film of moisture for 12-96 hours. Zoospore germination occurs in the temperatures (15-30°C), with an optimum at 22-25°C. Sporangia and spore of the pathogen are disseminated by wind, rain and animals. The pathogen infects crab grass. Zinc deficiency predisposes plants to infection.
i. Cultural Practices: Several cultural practices reduce severity of diseases. These are designed to eliminate the causal pathogens from a particular area, to significantly reduce primary inoculum or to stimulate growth during the first month after planting.
ii. Sow the seed before rainy season begins.
iii. Fungicidal Management: Metalaxyl (Ridomil) can be applied to seed, in furrows as granules or sprayed on foliage. Seed application (Apron 35 W.P., at the rate 0.25 per cent (2.5 g/kg seed) in a slurry is most economical and total control can be obtained. Systemic fungicides usually act on but one site in the fungus, whereas protective (non-systemic) fungicides act on several sites.
Thus in the former case only one change in the fungus is required to develop tolerance.Treatment of seed with apron 35 w.p. at the rate 0.25 per cent is a good safeguard against any possible seed-borne infection.
Spraying of fungicides such as mancozeb at the rate 2.5-3.0 per cent as soon as disease symptoms appear to protect valuable and breeding materials. Sun drying of seeds lead to inactivation of mycelium present in seed and also reduce moisture levels. Seeds with less than 15 per cent moisture content produce healthy plants.
iv. Use only disease resistant hybrids and composites in disease prone areas.
17. Polysora Rust Disease:
Among the rust diseases in maize Polysora rust or tropical rust or southern corn rust (Puccinia polysora Underw) is an important disease in tropical areas. It occurs in many parts of the world, and it is a recent introduction in the peninsular India in 1991 (Karnataka and Tamil Nadu during rainy season and Coastal districts of Andhra Pradesh during winter) on certain maize cultivars in Mysore distric.
In recent years, the incidence of P. polysora has taken a heavy toll in majority cultivars grown in Karnataka namely Mysore, Mandya, Hassan, Kolar, part of Coorg, Shimoga and Chitradurga district. Polysora rust is a warm weather disease favoured by wet weather infection and disease development at 12- 27°C temperature. Rain drizzle or even heavy dews allow disease formation.
Development on lower surface is more as compared to upper surface. The telia are circular to elongate, 0.2-0.5 mm in diameter dark chocolate brown to black and remain covered by the epidermis longer then the common rust. Telia often appear in circles around the uredial pustules.
The teliospores are dark brown, smooth angularly ellipsoid or oblong, rounded at both the ends and 18-27 x 29-41 µm. They are brittle, usually two celled constricted at the septum and born on the short 10-30 pm persistant brownish pedicels one fourth length of the spore. Infected maize leaves become chlorotic and dry.
It differs from common rust (P. sorghi) in pustule size, shape and colour however the most pronounced variation is that it kills the host unlike P. sorghi.
Raid (1988) found that even if infection comes as late as after anthesis, losses occur and the pathogen may cause heavy losses when the environmental conditions are conducive for disease development. However, Zummo (1988) reported that heavy losses may occure due to this disease if infection occurs an early stages.
The pathogen has the potential of being destructive if infection comes after anthesis. Melching (1975) reported yield loss up to 37% due to P. polysora and rated this disease as the most destructive among the three rusts of maize.
ADVERTISEMENTS:
Ardon (1988) recorded yield loss up to the tune of 45% especially on late planted maize however, Frederekson (1990) reported 60% loss in grain yield. Severe losses due to this disease can occur especially if infection occurs early.
The presence of Polysora rust in the peninsular India, particularly in states like Karnataka, could have an adverse impact on maize production. It is also expected that the spread of the disease to new locations could pose a major threat to maize cultivation, as this is considered to be the most destructive amongst the rust of maize.
Polysora rust is a warm weather disease favoured by wet weather infection and disease development at temperatures (27°C) and high relative humidity. Disease develops rapidly in warm weather and uredospores comprise both primary and secondary inoculum. Teliospores of P. polysora are rare and are not known to be germinated therefore they are unimportant in disease cycle.
The uredospores constitute both primary and secondary inoculum and are carried to maize plants by wind or on infected materials. No alternate host for this disease has been found.
Highly resistant genotypes have smaller uredosori than the moderately resistant or susceptible ones. Resistant lines normally show small chlorotic or necrotic flecks with no sporulation.
Maize lines with small pustules surrounded by chlorotic or necrotic zone were rated as resistant while well developed pustules were considered susceptible. Ullstrup (1965) reported a dominant gene of resistance and designated it as Rpp9.
A major gene on chr. 10S accounted for 83% of phenotypic variation for polysora rust resistance in a mapping population based on cross between tropical x temperate lines, with the tropical inbreds as a sources of resistance to polysora rust. RFLP markers flanking this locus have been identified.
Another study reported in a different genetic background, a QRL (Quantitative resistant locus) to P. polysora on chr. 2 and SSR markers closely linked to this locus.
Brewbaker (2005) demonstrated that synthetic MIRSYN3 based on 19 highly resistant inbreeds showed resistance through 6 cycles of recurrent selection. Jines (2006) prepared a multiple interval mapping model, including four QTL, accounted for 88% of the variation among average disease ratings.
A major QTL located on the short arm of chromosome 10, explained 83% of the phenotypic variation, with the NC300 allele carrying the resistance. They also stated that the maturity and Polysora rust rating were slightly correlated, but QTL for the two traits did not co-localize.
These studies clearly indicate that a few major loci undergrid general resistance to polysora rust in tropical maize germplasm. A net work project is going on at Directorate of maize Research on gene pyramiding on Polysora rust and further studies are in progress.
Based on the work carried out the management strategies for the Polysora rust is given below:
i. A fungicide application is useful when pustules first appear on the leaves.
ii. Three sprays of Dithane M-45 beginning from first appearance of symptoms at 15 days interval can minimize the disease.
iii. Use resistant varieties. The sources of resistance are; NAI 112, SKV 18, SKV 21, NAH 2049 (Nithyashree) a resistant hybrid to Polysora rust, TLB and SDM has been released in Karnatka.