ADVERTISEMENTS:
The following points highlight the top six experiments on osmosis in plants. Some of the experiments are: 1. Demonstration of the Phenomenon of Osmosis 2. Demonstration of Osmosis by Osmoscopes 3. Demonstration of Plasmolysis and Determination of Isotonic Conc. of the Cell Sap 4. Determination of Osmotic Pressure of Integrated Plant Tissues and Others.
Experiment # 1
Demonstration of the Phenomenon of Osmosis:
Experiment:
ADVERTISEMENTS:
A small funnel is taken and its broad mouth is closed with a piece of parchment or egg membrane. It is then completely filled with 1 ml glucose solution (180 06gm/litre). The nose of a 10 ml pipette is fitted with the stem of the funnel with the help of rubber tubing.
The level of the sugar solution is brought to a visible mark by adding more sugar solution drop by drop through open end of the pipette. The apparatus is then placed over a beaker containing pure water and clamped properly. The increase in the level of sugar solution is noted at definite intervals.
Observation:
ADVERTISEMENTS:
The level of sugar solution increases in the pipette gradually. The rate of this increase declines with time. A positive test for glucose (a brick red ppt.) is obtained when an aliquot of water from the beaker is tested with Fehling solution (Mix Fehling A containing 35gm GUSO4 plus 500 ml water, and Fehling B containing 50gm NaOH plus 173gm Rochelle salt plus 500 ml water, in equal proportions, add the test solution to it and heat strongly).
Inference:
The following inferences can be drawn from this experiment:
(a) The sugar solution rises in the pipette because of accumulation of water molecules which pass through the semipermeable membrane due to endosmosis.
(b) The accumulation of water dilutes the osmotic cone, of sugar solution. Hence the rate of increase of the level of sugar solution inside the pipette decreases with time.
(c) The positive reaction of sugar in the water of the beaker indicates that some sugar molecules have also come out through the membrane by exosmosis as the membrane is not truly semipermeable but differentially permeable.
Experiment # 2
Demonstration of Osmosis by Osmoscopes:
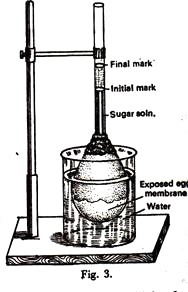
A. Egg osmoscope:
Experiment:
ADVERTISEMENTS:
The inner contents of an egg are taken out through a small hole made at one end of it. Tb obtain the semipermeable membrane, about one-third of the shell is immersed in conc. HCL very carefully.
The acid dissolves the shell (made up of CaCO2) exposing the inner membrane of the egg. It is then washed well with water without damaging the semipermeable membrane. The nose of a one milliliter pipette is inserted through the hole of the shell up to some distance avoiding contact with the membrane, and sealed with sealing wax or lacre.
The egg is filled with a strong solution of 1 M sucrose (342.30gm/litre) through the open end of the pipette and the level is brought to a visible mark on the pipette which is noted. Now, the egg membrane of the setup is immersed in a beaker of pure water and clamped vertically (Figure 3).
Observation:
ADVERTISEMENTS:
After some time the liquid inside the pipette rises. This rise declines with time.
Inference:
As in Expt. 3 (i).
ADVERTISEMENTS:
N.B. When the egg osmoscope is placed in an isotonic medium, there will be no rise of liquid in the pipette; in a hypotonic medium the level will rise due to endosmosis; and in a hypertonic medium the level of liquid will fall due to exosmosis.
B. Potato osmoscope:
Experiment:
A large potato tuber is first skinned and cut into a rectangular shape. A simple well is made at the centre of the tuber with the help of a cork borer and scalpel without piercing the other side. This potato osmoscope is then half-filled with 1 M sucrose solution; its level is marked with a pin and is placed in a petridish containing pure water.
ADVERTISEMENTS:
Observation:
After some time the level of liquid rises in the osmoscope.
Inference:
As in Expt. 3 (i). Here the potato tissue is acting as a semipermeable membrane.
N.B. The potato osmoscope may be placed in isotonic, hypotonic and hypertonic solutions and the corresponding changes in the level of liquid in the osmoscope may be observed.
Experiment # 3
Demonstration of Plasmolysis and Determination of Isotonic Conc. of the Cell Sap:
Experiment:
ADVERTISEMENTS:
Epidermal peelings from the lower surface of Rhoeo or Tradescantia leaves are suitably employed in this experiment because the protoplasm of the cells is clearly visible due to presence of anthocyanin pigment. Spirogyra filaments may also be used in this experiment.
0.15, 0.20, 0.25, 0.30, 0.35 and 0.40 M sucrose solutions are prepared from 1 M solution. 2 ml of each is poured in separate watch glasses and a few peelings are immersed in each of the solutions.
After about 40-50 minutes one peeling is taken out from each solution, mounted in the same solution and examined under the microscope to study the number of cells plasmolysed under the field of vision.
Observation:
Two concentrations are found out, one where plasmolysis has just started (i.e., slightly stronger than the cell sap) and the next lower concentration which just fails to plasmolyse the cells.
ADVERTISEMENTS:
Average of the two concentrations gives approximately the equivalent concentration of the cell sap. The extent of plasmolysis (both shrinkage of protoplasm and number of cells plasmolysed) also varies in different molar solutions.
Inference:
Plasmolysis of the cells does not occur in molar solutions of sucrose which are hypotonic and isotonic to the cell sap. Plasmolysis occurs only in case of hypertonic solutions. Incipient plasmolysis occurs at a concentration which is just above the isotonic concentration.
N.B. It is not possible to determine the exact concentration at which incipient plasmolysis (turgour pressure is zero) occurs. Thus the average of the concentrations mentioned above is taken as isotonic concentration. In case of individual cells, this method of determination of isotonic conc. should be adopted.
In case of integrated plant tissue, isotonic concentration may be obtained by taking the average of two concentrations of sugar solution where plasmolysis has just started and where cent percent cells plasmolysed. This concentration can be directly determined from a curve (percentage of cell plasmolysed/concentrations of sugar solutions) taking the concentration to be isotonic where 50% of the cell plasmolysed (Figure 4).
When the plasmolysed cells are put in pure water, deplasmolysis takes place that is protoplasm regains its original shape. If the concentration of the sugar solution is too strong, no deplasmolysis will take place indicating death of protoplasm.
This experiment may be performed with coloured flower petals, filamentous algae like Spirogyra, etc. The pattern of shrinkage of protoplasm is species of plant.
Experiment # 4
Determination of Osmotic Pressure of Integrated Plant Tissues:
Experiment:
Lower epidermal peelings of Rhoeo, Tradescantia or filamentous algae like Spirogyra may be used for this experiment. The isotonic concentration may be determined, as in Expt. 3 (ii).
Results:
Osmotic pressure of the cells is calculated by either of the following formulae:
Discussion:
Osmotic pressure thus determined gives the approximate value since the accurate concentration of the cell sap cannot be determined using plasmolytic method due to several reasons.
Some of the important sources of error are as follows:
(a) For normal and ordinary cells the actual isotonic conc. of the cells can only be obtained by correcting for the changes in volume of the cells associated with changes in osmotic conc. (see N.B.).
(b) Adhesion of protoplasm to the cell wall in certain types of cells may result in excessive osmotic pressure values. To get an accurate osmotic pressure, the calculated pressure must be multiplied by the ratio of the normal volume to the minimum volume of the cell, i.e., at the stage of plasmolysis (see N.B.).
(c) The exact determination of incipient plasmolysis is difficult because the very initiation of the separation of the protoplasm from its wall cannot be observed clearly.
(d) The sugar solutions may change the permeability of the cell wall bringing about considerable errors.
(e) Cell wall may be impermeable to the plasmolysing external solutions and may get twisted.
(f) Abnormal conditions of the protoplasm may result from mechanical shock due to cutting or isolation of tissues.
(g) The intact cells may be adversely affected by the sap released from the cut cells.
(h) Elastic nature of the cell wall may lead to inaccurate results.
N.B. The volume correction is made as follows:
C = Ci (Vi/V)
Where
C = conc. at normal condition,
Ci = conc. where plasmolysis has just started,
V = volume at normal condition (integrated cells),
Vi = volume at conc. where plasmolysis has just started
Since measurement of volume is impossible due to irregular shape of the cells, the average area (Length × breadth) may be taken into consideration.
Osmotic pressure may also be determined by cryoscopic method.
The following equation relating to freezing point depression and osmotic pressure may be employed:
O.P = 22.4 × A/ 1.86
Where A is the freezing point depression.
A is determined as follows:
1 molar solution of a nonelectrolyte freezes at a temperature of -1.86°C and its theoretical O. P. is 22.4 atmospheres. For actual determination of A of cell sap, the cell sap at first must be extracted by killing the cells by freezing.
The vessel containing the experimental tissue is surrounded with a mixture of ice and common salt. The frozen tissue is then allowed to melt. The sap can now be easily extracted with mere hand press. The freezing point of the macerated material may be determined.
Molecular weight of a substance may be calculated from the following formula, when strength of the solution in per cent or gm/litre, osmotic pressure and room temperature are known.
PV = (g/M) X RT
Or, M = g R T/P V
Where
g = gm of substance,
R = universal gas constant (0.082),
T = 273 + laboratory temperature, P osmotic pressure,
V = volume in litre,
M= molecular weight of the substance.
Integrated tissue like potato tuber may also be taken for this experiment of osmotic pressure determination. Here, isotonic concentration can be obtained by taking the concentration of sugar as isotonic where no change in weight takes place.
Experiment # 5
Determination of Mean Suction Pressure or Diffusion Pressure Deficit of Plant Cells:
Experiment:
20 ml each of 0 15, 0-20, 0-25, 0-30, 0 35 and 0.40 molar concentrations are prepared from 1 M stock solution of sucrose. All suberized peripheral layers from a fresh potato tuber or beet root are removed and cut into 1 cm x 2 cm pieces using a cork borer and a scalpel.
Each piece is washed in distilled water, blotted well, weighed (initial weight) and transferred to each of the graded solutions of sucrose. These are then left for about an hour. After the stipulated period the pieces are taken out, blotted carefully and reweighed (final weight).
In the above way, the osmotic concentration of fully turgid potato tuber tissue (made turgid by keeping the tissue in water for half an hour previously) and that of partially dehydrated tissue (made partially dehydrated by keeping the tissue in open air for half an hour) are separately determined.
The difference between the osmotic pressures obtained in case of dehydrated and normal tissues and that between normal and turgid tissues give the D.P.D), of dehydrated and normal tissues respectively.
Results:
The percentage increase or decrease in weight of the tissue i determined for each concentration of sucrose solution. Results are plotted on a graph paper taking sugar concentrations as abscissa and the percentage change in weight as ordinate. The concentration of sucrose corresponding, to no-change-in-weight is found out from the graph.
This value gives the osmotic concentration of the cell sap when osmotic pressure is equal to the suction pressure (D.P.D.), turgour pressure being zero. The D.P.D. is calculated by applying the formula of osmotic pressure given in Expt. 3 (iv). The necessary temperature and volume corrections may also be made.
Discussion:
The D.P.D. is obtained here indirectly because the D.P.D. of a solution is equal to its osmotic pressure at the isotonic concentration of the cell sap, i.e., at an equilibrium point.
This value is obtained from the molar strength of the sucrose solution with which cells or tissues come to equilibrium at incipient plasmolysis, and when the cell volume is at its relative minimum due to elimination of turgour pressure brought about by exosmosis of water.
The suction pressure values may be identical with osmotic pressure values only if the cells are initially at the incipient plasmolysis.
Experiment # 6
Determination of Turgour Pressure of Plant Tissue:
Experiment:
The isotonic concentration of a suitable plant tissue is determined according to Expt. 3 (iii) or 3 (v), whichever is convenient. The osmotic pressure of the tissue (which is here equal to the suction pressure or D.P.D., the turgour pressure being zero) is first determined. This is taken as the osmotic pressure (P) of the cell sap at that stage.
The tissue is then kept in pure water to allow it to increase in turgour pressure. After 10 minutes the tissue is taken out and osmotic pressure (suction pressure or D.P.D., turgour being zero) is similarly determined. The process is repeated until the suction pressure reaches a constant value and corresponding suction pressures are recorded.
Results:
Turgour pressure is obtained by the formula,
T = P —S where P = osmotic pressure of the cell sap (taken as constant), S = suction pressures at different time intervals, and T = turgour pressure at a particular time. Thus difference between the initial osmotic pressure (P) and that determined at every 10 minutes interval (S) gives the rate of increase in turgour pressure (T).
Discussion:
When a solution is confined within a semipermeable membrane immersed in water, there will be a net movement of water molecules through the membrane, because the diffusion pressure of water molecules is greater on water outside than that of the solution inside the membrane.
The inward passage of water thus develops turgour pressure within the cell. As the turgour pressure increases, suction pressure (D.P.D.) falls. In the present experiment the initial osmotic pressure of the cell has been taken as its maximum osmotic pressure and at isotonic concentration of the external solution this equals the D.P.D. of the cell (turgour being zero).
When immersed in pure water again, the turgour pressure increases due to endosmosis and as a result the suction pressure falls. The osmotic pressure determined at this stage using isotonic concentration (which must be less than the initial value) gives the suction pressure.
This value subtracted from the initial osmotic pressure value, gives turgour pressure at that particular suction pressure of the tissue. When the maximum turgour pressure of the cell has reached, no further change in suction pressure takes place.