ADVERTISEMENTS:
Process of Diffusion in Plant Cell (With Diagrams)!
Diffusion:
The movement of various substances into a plant, usually from the soil, out of which the green plant synthesises the numerous complex organic compounds, is accomplished, principally through the agency of the process known generally as diffusion.
In some cases, however, the operation of the diffusion phenomena is complicated by other factors.
ADVERTISEMENTS:
We know that in higher green plants, some substances enter the living cells through the aerial organs—the diffusion of CO2 and O2 from the atmosphere into the plants is principally through stomata. From soil, water and ions of simple inorganic salts pass into the plants through the root cells by a process which is basically diffusion, though greatly modified by other factors.
Similarly, the loss of large quantities of water as vapour from leaves and other aerial organs into the atmosphere is also accomplished by diffusion. O2 evolved in photosynthesis and the respiratory CO2 also diffuse out of the plant into the atmosphere. The exhaled CO2 in respiration of the root cells diffuses out into the soil.
The movement of substance within a plant, i.e., from one part of a plant to another, is mainly by diffusion. The gases move through the intercellular spaces and the water and solutes through the dead and living vessels and also from cell to cell by diffusion, each following its own independent diffusion pressure or concentration gradient.
There are very few, if any, physiological processes occurring in plants which do not directly or indirectly involve the diffusion phenomena. What is diffusion then? According to the kinetic theory, the molecules or ions of all substances (gases, liquids, solutes or solids) are in constant motion in all directions, which is due to their individual kinetic activity.
ADVERTISEMENTS:
This is called diffusion. In a mixture of several substances separated by a membrane, the molecules or ions of each substance will diffuse in that direction which is from a region of its own higher concentration or pressure to its own lower concentration or pressure or in other words from a region of its own higher activity to one of its own lesser activity of the particular substance concerned.
And the direction of this diffusion is independent of the direction of diffusions of ions or molecules of other substances present. From plants, the outward diffusing water vapour molecules in transpiration or oxygen evolved in photosynthesis do not carry respiratory CO2 with them, but each gas diffuses independently of the other, depending only on the difference of its own concentration or pressure, inside and outside the plant.
Diffusion of Gases:
It is well known that when a gas is allowed entry in a room, the molecules of the gas, owing to their kinetic energy, become evenly distributed throughout the room. If a bottle of chloroform or ether is opened indoors, the distinctive odour can be perceived in all parts of the room within a short time.
This is diffusion of gases and the rate of diffusion is dependent upon the concentration of the particular gas, the temperature, the density and also to some extent on the presence of other gases.
Diffusion of Dissolved Substances (Solutes):
If a blue crystal of CuSO4 is dropped in a beaker containing water, the diffusion of the dissociated ions of Cu++ and SO4= or entire molecules of CuSO4 can be observed by the slow change in the colour of water.
This rate of diffusion of solute molecules or ions through the solvent is extremely slow and may principally be due to the fact that the densely packed particles of water (solvent) considerably impede the diffusion of particles of the solute CuSO4.
The direction of diffusion of a particular Solute ion of molecule follows the same general pattern, i.e., from higher concentration or pressure to its lower concentration or pressure, regardless of diffusion, of other solute particles, if any, in the same system. The rates of diffusion are also generally controlled by principles which are essentially similar to those governing diffusion of gases.
Diffusion Through a Membrane:
ADVERTISEMENTS:
Diffusion takes place also through membranes. Three types of membranes are possible, permeable, impermeable and semipermeable. Permeable membranes are those which allow diffusion of both solvent and solute molecules or ions through them. Ordinary cellulose cell walls of young non-vacuolated cells are examples of completely permeable membranes.
Lignified cell walls are also quite freely permeable to water and when wet, to solute ions and molecules as well. Impermeable membranes are those which prohibit the entry of both solvent and solute particles.
Suberised or heavily cutinised cell walls in plants are practically impermeable as regards the passage of water and dissolved substances. Semipermeable or differentially permeable membranes are those which allow the diffusion only of the solvent particles through them while restricting the entrance of the solute particles, i.e., the membrane is permeable to one substance but impermeable to the other.
In this connection, the only solvent we need to consider is water, for water is the only important liquid in living organisms which moves from cell to cell and hence all our further discussions of this phenomenon will naturally be in terms of water and aqueous solutions.
ADVERTISEMENTS:
If a U tube (Fig. 663A) is divided by a permeable membrane, filled on one arm with pure water and the other with a solution of sucrose, the molecules of sucrose will diffuse through the permeable membrane towards water because the diffusion pressure and concentration of sucrose molecules are higher on the right-hand than on the left- hand side and the water molecules will move in the opposite direction for its diffusion pressure or concentration is higher in the left portion than in the right.
Thus there will be a thorough mixing of both sucrose and water molecules in the two arms of the tube. An equilibrium is eventually established between the two arms of the tube when equal quantities of sucrose and water molecules are present on the two sides of the partition.
If the membrane is more rapidly penetrated by one of the two substances that substance will at first pass more quickly than the other but ultimately equalisation of the concentration and the pressure of the solutions in the two arms must result.
The case will be very different, however, when the partition, separating the two liquids is semipermeable, i.e., permeable only to the molecules of water while molecules of sucrose are quite unable to pass through it (Fig. 663C).
ADVERTISEMENTS:
The result will be that owing to greater diffusion pressure (or concentration) of water in the left arm of the U tube, water molecules will pass through the membrane towards the right arm. Volume for volume, the number of molecules of water is certainly higher in the left arm than in the right arm since in the solution of sucrose, a large proportion of molecules of water are replaced by sucrose molecules.
Thus there will be a greater accumulation of water molecules on the side of the membrane containing sucrose solution and the level of solution in the right arm will consequently increase considerably.
This diffusion of water molecules through a semipermeable or differentially permeable membrane from the region of higher concentration and higher diffusion pressure of water to a region where it is lesser is known as osmosis and the pressure developed as a result of this is known as osmotic pressure.
It must be pointed out here, however, that semipermeable membranes which completely restrict the movement of solute particles or will completely restrict the movement of water molecules from the right arm to the left arm of the U tube, are only theoretically possible.
ADVERTISEMENTS:
In practice, however, most semipermeable membranes are really only differentially permeable and allow passage to some sucrose molecules or even to some solvent (water) molecules from the right to the left of the tube. In U tube B, where the membrane is impermeable, there is theoretically no net diffusion of either the solvent or the solute molecules.
Obscurities have sometimes resulted from the application of the term osmosis to the movement of solutes through the membranes. The use of the term in this sense has not only led to much misunderstanding but the concepts of many students have remained vague as regards the process of absorption of water and absorption of solutes which two terms, they are apt to consider synonymous.
The movement of solutes through a membrane, though certainly primarily a process of diffusion, may even be simple diffusion, but complicated enormously by a number of physico-chemical factors which do not play any part in the process of diffusion we know of.
According to the principles of diffusion, the molecules or ions of all substances in solution, gases or even dispersed particles in a colloidal system, tend to attain an equal distribution in terms of concentration through all parts of a system. The osmotic movement of water is in response to the same general principles; equal distribution is, however, seldom attained in living organisms.
Semipermeable membranes and the process of osmosis play the primary role in the absorption of water by vegetable cells. In studying the phenomena and the laws governing them, consideration of natural vegetable cells may be deferred for a while, and the study of the operation of the osmotic phenomena and the effects produced in an artificial cell with an artificially prepared synthetic membrane containing an osmotically active substance, might give us a clearer understanding of the process that actually takes place in a living cell.
ADVERTISEMENTS:
An artificial osmotic chamber with a true semipermeable membrane can be prepared easily by taking a clay cell of galvanic elements coated on the inside with copper ferrocyanide. Copper ferrocyanide is prepared by mixing CuSO4 with potassium ferrocyanide.
Another type of artificial semipermeable membrane can be obtained by mixing a solution of gelatine with tannic acid.
The naturally occurring semipermeable membranes include bladders of fishes and animals, the thin white membrane in eggs when the hard outer calcium coatings are removed by dissolving in acids and the most important of all, the plasma membranes of living vacuolated mature cells of the vegetable tissue. The plasma membrane is the semipermeable membrane in plant cells.
It is the presence of this differentially permeable membrane which gives to osmosis its distinctive aspect as compared with other diffusion processes.
How can you distinguish between a living and a non-living cell? Sometimes, the streaming movements of the protoplasm show us that a cell is living. Another method is by staining a cell with a dye such as neutral red or methylene blue.
These dyes only stain the cell contents in the vacuole and may accumulate there. Non-living cells, or living cells when killed never show this type of staining. A third characteristic of the living cells is that their cell contents can be shrunk or plasmolysed away from the cell wall when placed in a solution whose concentration is greater than that of the cell contents.
ADVERTISEMENTS:
The cell contents thus can be separated from its surrounding cell wall. Obviously then, even the living plant cell consists of a non-living part (cell wall) and a living part (protoplasm). The non-living cell wall, however, has pronounced physiological significance since it markedly affects the protoplasm, particularly its water content and growth.
The most important part of a cell is this living protoplasm for here are synthesised and broken down all the organic chemical substances from which life itself is regenerated. Energy is absorbed in synthesis and released in the breakdown.
We are concerned here with the activities of living cells which certainly depend upon the properties of the living protoplasm. The nature of the protoplasm can, however, be only properly understood if the chemistry and the physics of the protoplasm are known to us.
A chemical analysis of protoplasm obtained from some lower plants such as slime molds, shows that as percentage of the fresh weight, water content varies from 80- 90%, proteins 7-10%, lipid substances 1-2%, other organic and inorganic materials, just over 1%.
The analysis is gross, and it certainly does not mean that new protoplasm could be formed just by mixing the above substances in the proportion indicated above. It only gives us some insight into the essential physical and chemical properties of protoplasm.
Among the many kinds of proteins in the protoplasm are the nucleoproteins. The nucleoproteins are perhaps the most fundamental substance of life as indicated by the fact that the smallest living entity (?) virus, consists almost entirely of nucleoproteins which are the bearers of heredity in all organisms—plants or animals.
The phospholipids (e.g., lecithin) are the main fatty substances of the protoplasm being mostly confined to the structural components of protoplasm, e.g., plastids, mitochondria, etc. They are also associated with the cell membrane.
All these structural components are suspended in the ground substance of the protoplasm which is made up predominantly of proteinaceous sol or gel. The ground substance is usually considerably viscous than the cell sap of the vacuoles and since viscosity and gel formations as we know are characteristics of a colloidal system, the protoplasm is obviously a colloid.
The non-living part of the cell wall also shows colloidal properties. The vacuolar cell sap also possess enough colloidal materials, e.g., tannins which hold dyes such as methylene blue. Optically, the cell sap, however, appears as homogeneous. The cell walls are made of colloidal gels of cellulose and other organic substances, e.g., pectin.
All the components of a living cell, the cell wall, vacuole (both non-living) and protoplasm contain large amounts of water which pass readily from the living to the non-living parts and vice versa.
The planes separating the three components of a living cell—the protoplasm, cell wall and the vacuole are known as interfaces. At each interface, special, physical forces such as surface tension, operate freely. The substances near the interfaces tend to be concentrated sometimes producing the phenomenon of adsorption.
Water movement across the cell membrane has usually been expressed in terms of diffusion pressures and their differences and osmotic pressure of solutions. However, it is more reasonable to explain this phenomenon in thermodynamic terms, since movement takes place only when energy is available in some form.
All molecules at any temperature above absolute zero have a free energy with which it has a tendency to move about in the surrounding space. As the number of molecules per unit space increases, the total free energy also increases; the free energy of Avo-gadro’s number (6.03 x 1023) of a chemical species present in 1 gram mole is termed the chemical potential of that species—whether in the molecular or in the ionic form.
In solutions both solute and solvent particles have chemical potentials. When we talk of chemical potential of water, we refer to it simply as water potential, usually designated by the Greek letter ¥ (psi).
When two solutions are separated by a permeable membrane both solute and solvent molecules diffuse across the membrane until the chemical potentials of both on two sides of the membrane are equal. Movement is always from a higher to a lower potential.
There is diffusion even after equilibrium is attained, but the rate of diffusion in both directions is equal. When the membrane is semipermeable, movement of only solute or solvent particles is permitted by the membrane. In the case of plant membranes only the solvent water molecules are permitted to pass.
Water molecules in pure water have the maximum chemical potential; however, when solute molecules are dissolved in it, this potential is decreased, the greater the number of such solute molecules, that is greater the concentration, less is the water potential.
As in the case of the Celsius (centigrade) scale of temperature measurement, where the freezing point of water is arbitrarily fixed to be zero, water potential of pure water is arbitrarily taken to be zero. Consequently the water potential- of any solution containing solute particles is negative.
The water potential of a 0.1 M solution is higher than a 0.2 M solution, because it is more dilute and its water potential is less negative. Water potential is usually expressed in terms of pressure, i.e., bars on atmospheres. Increase in temperature or pressure increases water potential. Water potential is also affected by substances in the environment which absorbs water.
Movement of water across membranes practically impermeable to solute particles’ is called osmosis. Osmotic pressure of a solution is a function of its solute concentration at a particular temperature according to the equation
π (pi) =CRT,
where π is the osmotic pressure, C the molar concentration (since pressure is the result of the total activity of the number of molecules), R is the Gas constant (0.082 litre atmospheres/mole degree or 0.0357 litre cal. per degree centigrade) and T is the absolute temperature. It is usually expressed in litre atmospheres.
Although osmotic pressure is a positive quantity, osmotic potential is always negative, since it is the value by which water potential is lowered when the solute is dissolved in pure water.
Two major components of water potential of a solution are pressure potential and osmotic potential. At a particular temperature water potential is the sum of pressure potential (ψр) and osmotic potential (ψπ) Pressure potential is the effect of atmospheric pressure or any other pressure applied.
Thus, pressure potential is the difference of water potential and osmotic potential. It may be positive or negative accordingly as pressure is applied or the cells are in a state of tension. Plant cells also contain many hydrophilic, colloidal gel-like and porous surfaces, e.g., the cell wall, which absorb and retain water with some tenacity; the water potential of this matrix is referred to as matrix potential, ψm.
If the colloidal matrix and the cell solution are two distinct phases in equilibrium with each other, then osmotic potential of the cell solution may well be equal and either value added to the pressure potential should constitute the water potential of the system. However, several workers consider them as separate entities and the total effect is believed to be additive according to the equation
ψ (psi)= ψp + ψπ + ψm
Water movement in plant cells is along a water potential gradient. Although it is primarily a diffusion process, the rates observed are much higher than what may be provided by a pure diffusion process.
There is evidence that there is a bulk movement of water. Presumably, the gradient in the transit region of the membrane surface is quite steep, so that the rate is considerably accelerated and water molecules are pulled in a bulk, somewhat as in the case of a siphon.
In most earlier text books water potential was considered as diffusion pressure of water and the difference in the water potentials of the external solution and cell solution as the diffusion pressure deficit or the suction pressure.
We shall now explain the process of osmosis particularly with reference to an osmometer using some of this terminology to familiarise the student with these concepts.
If an osmometer, as the artificial cell mentioned before is called (or simply osmotic chamber), containing a strong solution of sucrose is immersed in a vessel containing pure water and a mercury manometer is attached to it as shown in Fig. 668, the rise of column of mercury in the manometer tube due to inward diffusion of water will denote the measure of the pressure of the solution inside the cell.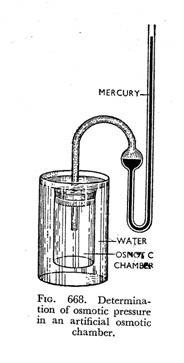 The artificial membrane is here rigid and as this membrane is permeable only to water, the inwardly directed diffusion of water from outside into the cell will cause a pressure to develop inside the membrane. The maximum pressure thus developed is quantitatively equal to the pressure on the solution to prevent any increase in its volume due to entrance of water.
The artificial membrane is here rigid and as this membrane is permeable only to water, the inwardly directed diffusion of water from outside into the cell will cause a pressure to develop inside the membrane. The maximum pressure thus developed is quantitatively equal to the pressure on the solution to prevent any increase in its volume due to entrance of water.
The inflow of water from outside (endosmosis) due to higher water potential there, causes a hydrostatic pressure to develop inside the cell. This hydrostatic pressure is directed against the cell wall and this actual pressure developed within the cell as a result of osmosis, is known as turgour pressure.
Actual pressure or turgour pressure developed during osmosis is seldom equal to osmotic pressure. If a solution, having an osmotic pressure of 10 atm. be immersed in a solution, having an osmotic pressure of 6 atm., water will diffuse inwards until at equilibrium, i.e., when the water potentials of the external and internal solutions are equal, the actual pressure developed, can at its maximum be only 4 atm.
Even if the external liquid be pure water, the actual pressure or the turgour pressure developed in the internal solution would still not be quite equal to its original osmotic pressure unless the membrane is completely inelastic. Thus in an osmometer, the maximum turgour pressure developed can only equal the osmotic pressure but can never exceed it.
When a solution is confined within a semipermeable membrane and the membrane is immersed in water, the passage of water through the membrane results in the development of turgour pressure on the solution side of the membrane.
But the maximum potential osmotic pressure which can develop in the solution must be equal to the excess of diffusion pressure of pure solvent over the diffusion pressure of water in the solution, but only when the solution is not under any pressure except, of course, atmospheric pressure.
In plant cells, other pressures must be taken into account in order to determine the suction pressure or diffusion pressure deficit (DPD) of the cell sap. Suction pressure is water absorbing capacity of the solution in the membrane.
For example, if a solution has an osmotic pressure of 10 atmospheres, it certainly means that the diffusion pressure of water in the solution is less than the diffusion pressure of pure water by 10 atm. or in other words, the suction pressure of such a solution is 10 atm.
Hence if a cell with cell sap solution of 10 atm. (suction pressure of also 10 atm.) is immersed in a solution of 4 atm. (suction pressure also 4 atm.) and is separated by a membrane permeable only to water, water will diffuse towards the region of its lesser diffusion pressure or greater diffusion pressure deficit (suction pressure), i.e., towards the solution whose osmotic pressure and suction pressure are both 10 atm.
If we disregard dilution for the present and the volume changes, the solution inside the membrane will exert a turgour pressure of 6 atm. and this will be the actual hydrostatic pressure developed within the cell.
As each action has an equal and opposite reaction, imposition of a pressure on the wall of the membrane will result in an out wardly directed wall pressure of 6 atm. The wall pressure evidently is always equal in magnitude to turgour pressure but acts in the opposite direction.
The wall pressure will necessarily increase the diffusion pressure of the enclosed solution of 10 atm. by 6 atm. Since the initial diffusion pressure deficit of the enclosed solution was also 10 atm., the development of a wall pressure of 6 atm., raises the diffusion pressure of water in the internal solution by 6 atm.
While at the same time reduces the diffusion pressure deficit of water in the internal solution to 4 atm. (10—6 = 4) which is the suction pressure or diffusion pressure deficit in the external solution.
Osmotic pressure is proportional to the number of particles of solutes per given volume of water under standard conditions of temperature and atmospheric pressure and irrespective of kind, weight or size of the particles.
If one membrane contains a solution with twice as many particles—molecules or ions—as that in the second, the first, evidently will have twice as much osmotic pressure as the second.
A solution, such as found inside the vacuoles of a mature plant cell containing a mixture of many solute molecules (or ions) will have the same osmotic pressure as a solution with a single salt if the ratio of the total number of dissolved molecules and ions to the number of water molecules is the same in each case.
In order to determine this proportional number of particles it is necessary to know the proportional weights of the particles. Since molecular weights give the proportional weights of the molecules, it is customary to use molecular solutions.
Molar solutions are solutions in which as many grams of solutes as its molecular weight are added to water to a final volume of 1,000 ml. Sucrose with an empirical formula of C12H22O11 has a molecular weight of 342 g; the molecular weight of glucose is 180 g, etc.
These amounts dissolved in 1,000 ml of water will give molar solutions (M) of sucrose and glucose respectively. A0.1 M solution of glucose will develop the same osmotic pressure as 0.1 M solution of sucrose as these two solutions will contain the same number of particles of molecules. Equimolecular solutions of non- electrolytes are thus iso-osmotic or isotonic.
A colloidal system should also possess an osmotic pressure, for osmotic pressure is determined by the proportion of the solute particles to solvent molecules and is independent of the kind and the size of particles.
The particles of the dispersed phase of a colloidal system theoretically then will have the same effect on the magnitude of the osmotic pressure as smaller molecules or ions. Large molecules or molecular aggregates theoretically have the same effect as smaller molecules or ions. Thus osmotic pressure, in a colloidal system, will depend upon the concentration of the particles of the dispersed phase.
As the total concentrations of the particles of the dispersed phase are relatively much smaller compared to particles in a true solution, all colloidal systems have very low osmotic pressure values, seldom exceeding a fraction of an atmosphere.
Sugars and complex organic substances are, as we know, non-electrolytes whereas the inorganic salts are electrically active, i.e., certain percentage of inorganic salts is dissociated when the salts are in solution. This increases the number of particles in a particular volume of water.
At a concentration 0.1 M NaCl, 80% of NaCl molecules are dissociated and therefore for every 100 NaCl molecules, 20 molecules will remain undissociated whereas 80 molecules are ionised into equal number of Na+ and Cl ions.
There will, therefore, be 180 particles either as molecules or ions, instead of 100 particles and the osmotic pressure would be 1.8 times that of a substance of the same molar concentration with only undissociated molecules. A 0.1 M solution of NaCl, therefore, will have an osmotic pressure approximately equivalent to 0.1 X 1.8 or 0.18 M solution of cane sugar or glucose or any other non-electrolyte.
The number which expresses by how much the osmotic concentration of an electrolyte is greater than an equimolecular solution of a non-electrolyte, is termed isotonic coefficient for that particular concentration; for 0.1 M NaCl, as we have seen, it is 1 .8.
The conditions affecting osmotic pressure are very similar to those which affect gas pressure and within certain limits are the same. General laws of vapour pressure also hold good for osmosis.
According to Avogadro’s hypothesis:
Equal volumes of all gases under identical condition of temperature and pressure, contain same number of molecules or particles. One molar weight of any gas at standard conditions (0°C. and 1 atm.) occupies 22.4 litres. If this gas be compressed to a volume of 1 litre, it will certainly exert a pressure of 22.4 atm. at 0°C. (From Boyle’s law) The osmotic pressure of a solution varies directly with concentration of the solution and inversely with the volume.
This theoretical value of 22.4 atm. sometimes slightly differs from actually measured pressure due to various reasons—24.83 atm. instead of 22.4. The theoretical value 22″4 atm. can be corrected for any desired temperature and pressure. (At 25°C., the osmotic pressure of 1 M solution is aproximately 27 atm.)
We shall again consider the case of an artificial rigid osmotic chamber with a membrane, permeable only to water (semipermeable membrane) and a long vertical glass tube of very small diameter attached at the open end as in Fig. 664.
Theoretically such a membrane, either inorganic or organic, should allow only the solvent molecules to pass through it, but as a matter of fact all artificial inorganic membranes— the same is true for organic as well—allow certain solute molecules to pass through them when certain conditions are fulfilled.
The osmotic chamber containing a 0.45 M solution of sucrose is immersed in pure water as in Fig. 664(A). Net movement of water inwards will take place by osmosis resulting in raising the column of water in the tube. At equilibrium, the suction pressure of water in the chamber will be zero, because of the imposition of a hydrostatic pressure (turgour pressure) of 10 atm. on the internal solution.
When equilibrium will be reached between osmotic chamber and the surrounding water, the level of solution in the vertical tube will remain stationary. What will be the maximum height of solution attained in the vertical tube at that condition? At this stage, the osmotic pressure of the solution in the chamber is equal to the actual pressure developed due to incoming water, i.e., turgour, which is also equal to 10 atm. at its maximum.
1 atmospheric pressure is equivalent to 76 cm of mercury and the specific gravity of mercury is 13.6. Thus the maximum height attained by the solution in the vertical tube attached to the osmotic chamber will be 76 x 10 x 13.6 cm or approximately 103 m. (We disregard here the dilution of the solution in the chamber and the changes in the volume.)
If the osmotic chamber is now taken out of water and then immersed in a solution of 0.25 M concentration as in Fig. 664(B), the level of column of water in the tube will certainly drop until the actual pressure developed on the membrane and the wall of the chamber is determined by the difference in concentration of the solutions inside and outside the chamber, i.e., approximately 4.48 atm. (22.4 x 0.2 =4.48 atm.).
The actual pressure developed (turgour) within the chamber can thus only attain a value of 4.48 atm. The suction pressure of the water in the solution inside the chamber (which was zero in pure water) will increase for it will now be subjected to a pressure of only 4.48 atm.
Imposition of a turgour pressure of only 4-48 atm. now will make the suction pressure equal to 10—4.48, i.e., 5.52 atm., equivalent to 0.25 M, which is exactly the concentration of the external solution. Thus the water level in the tube will be stationary at a height of 4.48 x 76 X 13.6 =46 m (this height is due to the actual hydrostatic pressure or turgour developed within the chamber, keeping the water column at that height).
If the chamber with its attached tube is again removed and transferred to an external solution of 0.15 M concentration, water will again start entering the chamber by endos- mosis and the turgour pressure will further rise and at its maximum will now be equal to 0.30 M (or 6.72 atm.).
The level of water in the tube will show a further rise until it reaches 6.72 X 76 x 13.6=69 m. Imposition of a turgour pressure of 6.72 atm. will College botany reduce the suction pressure of the solution inside to 3.28 atm. which is exactly the suction pressure of water in the external solution (0.15 M=3.28 atm.).
If the chamber is now finally transferred to an external solution, having the same concentration as that in the chamber (0.45 M), water will come out again by exosmosis until the levels of water column in the tube and in the external solution are exactly the same.
The turgour pressure will be progressively reduced to zero, and all the hydrostatic pressure developed on the walls of the chamber is thus released due to water exit.
And as the suction pressure of water in the solution inside the chamber is progressively released from the imposed turgour pressure, the suction pressure value progressively increases until it attains a maximum of 10 atm. (0.45 M) equal to the suction pressure of the external solution.
There is no net movement of water into the chamber when the chamber is thus brought into equilibrium with external solution—movement of water molecules certainly there is, but the rates of exosmosis and endosmosis are equal.
Dialysis:
Dialysis is essentially a sort of filtration in which the pore size of the membrane permits the diffusion of smaller particles from inside outside and vice versa, but larger molecules are retained.
Thus coenzymes can be separated from apoenzymes by dialysis. If the enzyme solution is taken inside a dialysis bag (collodion or other similar membranous structures) and the contents equilibrated with a buffer solution or water; the coenzyme molecules and metal ions which are small, pass out of the long but the larger protein molecules are retained within the bag.
Several changes of the outside solution are required, so that the diffusion of the smaller particles is complete or almost complete. If the outside solution is not changed, after sometime equilibrium is attained and the small particles are present both inside and Outside the Dialysis Bag or Tubing. Kidney Of Man And Animals Is An Efficient Dialyser.


