ADVERTISEMENTS:
Let us make an in-depth study of the mechanism of absorption of mineral salts from soil by plants.
Mechanism of Mineral Salt Absorption:
Previously, it was thought that the absorption of mineral salts from the soil took place along with the absorption of water but it is now well established that the mineral salt absorption and water absorption are two different processes. Mineral salts are absorbed from the soil solution in the form of ions. They are chiefly absorbed through the meristematic regions of the roots near the tips.
However, some mineral salts may also be absorbed at other locations on the root surface or over the entire root surface including zone of elongation and root hairs that depends upon the high availability of such minerals around them and/or strong tissue demand at such locations.
ADVERTISEMENTS:
Plasma membrane of the root cells is not permeable to all the ions. It is selectively permeable. All the ions of the same salt are not absorbed at equal rate but there is unequal absorption of ions. First step in the absorption of mineral salts is the process of Ion-Exchange which does not require metabolical energy but greatly facilitates mineral salt absorption.
Ion-Exchange:
The ions adsorbed on the surface of the walls or membranes of root cells may be exchanged with the ions of same sign from external solution. For example, the cation K+ of the external soil solution may be exchanged with H+ ion adsorbed on the surface of the root cells. Similarly, an anion may be exchanged with OH– ion. There are two theories regarding the mechanism of ion exchange:
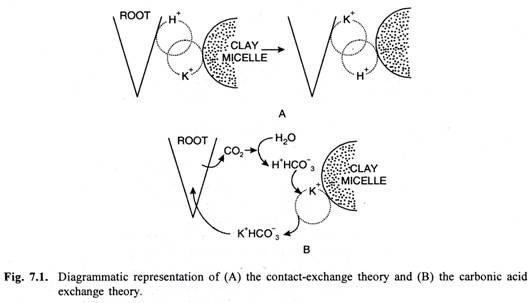
(i) Contact Exchange Theory:
According to this theory, the ions adsorbed on the surface of root cells and clay particles (or clay micelles) are not held tightly but oscillate within small volume of space. If the roots and clay particles are in close contact with each other, the oscillation volume of ions adsorbed on root-surface may overlap the oscillation volume of ions adsorbed on clay particles, and the ions adsorbed on clay particle may be exchanged with the ions adsorbed on root-surface directly without first being dissolved in soil solution (Fig. 7.1 A).
(ii) Carbonic Acid Exchange Theory:
According to this theory, the CO2 released during respiration of root cells combines with water to form carbonic acid (H2CO3). Carbonic acid dissociates into H+ and an anion HCO3– in soil solution. These H+ ions may be exchanged for cations adsorbed on clay particles.
The cations thus released into the soil solution from the clay particles, may be adsorbed on root cells in exchange for H+ ions or as ion pairs with bicarbonate (Fig. 7.1 B). Thus, soil solution plays an important role in carbonic acid exchange theory.
The further process of the absorption of mineral salts may be of two types:
(1) Passive and
(2) Active
(1) Passive Absorption of Mineral Salts:
When the concentration of mineral salts is higher in the outer solution than in the cell sap of the root cells, the mineral salts are absorbed according to the concentration gradient by simple process of diffusion. This is called as passive absorption because it does not require expenditure of metabolic energy.
It is now known that during passive absorption, the mineral salts may diffuse through cell membranes directly through lipid bilayer but mainly through trans-membrane ion-selective protein channels or trans-membrane carrier proteins. Carrier or channel mediated passive transport of mineral salts across the membrane is also called as facilitated diffusion (Fig. 7.6).
ADVERTISEMENTS:
(2) Active Absorption of Mineral Salts:
It has often been observed that the cell sap in plants accumulates large quantities of mineral salts ions against the concentration gradient. For example in alga Nitella the cell sap accumulated K+ and phosphate ions to such an extent that their concentrations were thousands and hundreds times greater than in the pond water in which the plant was growing.
This cannot be explained by simple diffusion or Donnan’s Equilibrium and has led people to believe that absorption and accumulation of mineral salts against the concentration gradient is an active process which involves the expenditure of metabolic energy through respiration.
Following evidences favour this view:
ADVERTISEMENTS:
(i) The factors like low temp., deficiency of O2, metabolic inhibitors etc. which inhibit metabolic activities like respiration in plants also inhibit accumulation of ions.
(ii) Rate of respiration is increased when a plant is transferred from water to salt solution (Salt Respiration).
It has now been accepted that active absorption of mineral salts involves the operation of a carrier compound present in the plasma membrane of the cells.
The Carrier Concept:
According to this theory the plasma membrane is impermeable to free ions. But some compound present in it acts as carrier and combines with ions to form carrier-ion-complex which can move across the membrane. On the inner surface of the membrane this complex breaks releasing ions into the cell while the carrier goes back to the outer surface to pick up fresh ions (Fig. 7.2).
Following observations strongly support the carrier concept of active absorption of mineral salts:
(i) Isotopic Exchange:
Several times, it has been found that actively absorbed radioactive ions (such as 35S04) cannot diffuse back or be exchanged with other ions in the outer solution indicating thereby that the plasma membrane is not permeable to free ions.
(ii) Saturation Effects:
ADVERTISEMENTS:
Beyond a certain limit, increased concentration of salts in outer solution does not bring about an increase in the rate of mineral salt absorption. It is because the active sites on the carrier compound become saturated with ions.
(iii) Specificity:
Active sites on carrier compound may be specific which can bind only some specific ions. This also explains the selective and unequal absorption of ions by the plants. There are two common hypotheses based on the carrier concept to explain the mechanism of active salt absorption, although they are not universally accepted.
(1) Lundegardh’s Cytochrome Pump Theory:
Lundegardh and Burstrom (1933) believed that there was a definite correlation between respiration and anion absorption. Thus when a plant is transferred from water to a salt solution the rate of respiration increases. This increase in rate of respiration over the normal respiration has been called as anion respiration or salt respiration.
The inhibition of salt respiration and the accompanying absorption of anions by CO and cyanides (which are known inhibitors of cytochrome oxidase of electron transport chain in mitochondria), later on led Lundegardh (1950, 54) to propose cytochrome pump theory which is based on the following assumptions:
(i) The mechanism of anion and cation absorption is different.
ADVERTISEMENTS:
(ii) Anions are absorbed through cytochrome chain by an active process.
(iii) Cations are absorbed passively.
According to this theory (Fig. 7.3):
(i) Dehydrogenase reactions on inner side of the membrane give rise to protons (H+) and electrons (e–).
(ii) The electron travels over the cytochrome chain towards outside the membrane, so that the Fe of the cytochrome becomes reduced (Fe++) on the outer surface and oxidised (Fe++) on the inner surface.
(iii) On the outer surface, the reduced cytochrome is oxidised by oxygen releasing the electron (e–) and taking an anion (A–).
ADVERTISEMENTS:
(iv) The electron thus released unites with H+ and oxygen to form water.
(v) The anion (A–) travels over the cytochrome chain towards inside.
(vi) On the inner surface the oxidised cytochrome becomes reduced by taking an electron produced through the dehydrogenase reactions, and the anion (A–) is released.
(vii) As a result of anion absorption, a cation (M+) moves passively from outside to inside to balance the anion.
Main defects of the above theory are:
(i) It envisages active absorption of only anions.
(ii) It does not explain selective uptake of ions.
(iii) It has been found that cations also stimulate respiration.
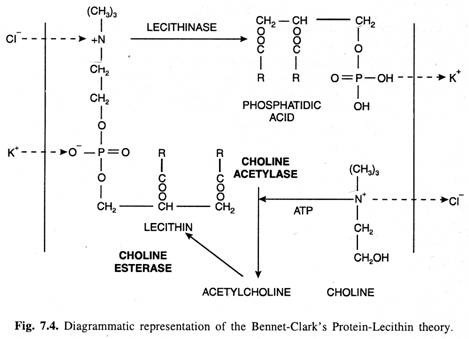
(2) Bennet-Clark’s Protein-Lecithin Theory:
In 1956, Bennet-Clark suggested that because the cell membranes chiefly consist of phospholipids and proteins and certain enzymes seem to be located on them, the carrier could be a protein associated with the phosphatide called as lecithin. He also assumed the presence of different phosphatides to correspond with the number of known competitive groups of cations and anions (which will be taken inside the cell).
According to this theory (Fig. 7.4), (i) the phosphate group in the phosphatide is regarded as the active centre binding the cations, and the basic choline group as the anion binding centre.
(ii) The ions are liberated on the inner surface of the membrane by decomposition of the lecithin by the enzyme lecithinase.
(iii) The regeneration of the carrier lecithin from phosphatidic acid and choline takes place in the presence of the enzymes choline acetylase and choline esterase and ATP. The latter acts as a source of energy.
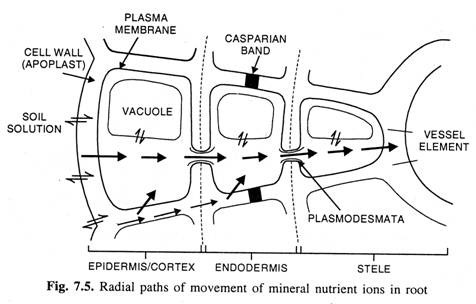
Once inside the epidermal cells of the root, the mineral salts in their ionic form move from one cell to another by:
(i) Apoplastic pathway (i.e., through cell walls and intercellular spaces),
(ii) Trans membrane pathway (i.e., by crossing the membranes) and
(iii) Symplastic pathway (i.e., through plasmodesmata), and ultimately reach to xylem vessels and tracheids (Fig. 7.5) from where they are carried to different parts of the shoot along with ascent of sap.