ADVERTISEMENTS:
The below mentioned article highlight the top two methods for culturing isolated pollen.
Method 1:
This method is described here for the culture of isolated pollen of tobacco.
This technique can be considered as the basic protocol for pollen culture and involves the following steps:
ADVERTISEMENTS:
Step 1.
Selection of suitable unopened flower bud, sterilization, excision of anther without filament are the same as described previously in anther culture.
Step 2.
About 50 anthers are placed in small sterile beaker containing 20 ml of liquid basal medium (MS or White or Nitsch and Nitsch).
ADVERTISEMENTS:
Step 3.
Anthers are then pressed against the side of the beaker with the sterile glass piston of a syringe to squeeze out the pollens (Fig 11.2).
Step 4.
The homogenized anthers are then filtered through a nylon sieve (pore diameter 40µ- 60µ) to remove the anther tissue debris.
Step 5.
The filtrate or pollen suspension is then centrifuged at low speed (500-800 revolution per minute) for 5 minutes. The supernatant containing fine debris is discarded and the pellet of pollen is suspended in fresh liquid medium and washed twice by repeated centrifugation and re-suspension in fresh liquid medium.
Step 6.
Pollens are mixed finally with measured volume of liquid basal medium so that it makes the density of 103-104 pollens/ml.
ADVERTISEMENTS:
Step 7.
2.5 ml of pollens suspension is pipetted off and is spread in 5 cm petridish. Pollens are best grown in liquid medium but, if necessary, they can be grown by plating in very soft agar added medium. Each dish is sealed with cello-tape to avoid dehydration.
Step 8.
Petridishes are incubated at 27-30° C under low intensity of white cool light (500 lux, 16 hrs.).
ADVERTISEMENTS:
Step 9.
Young embryoids can be observed after 30 days. The embryoids ultimately give rise to haploid plantlets.
Step 10.
Haploid plantlets are then incubated at 27- 50°C in a 16 hrs. day light regime at about 2,000 lux. Plantlets at maturity are transferred to soil as described in anther culture.
Method 2:
ADVERTISEMENTS:
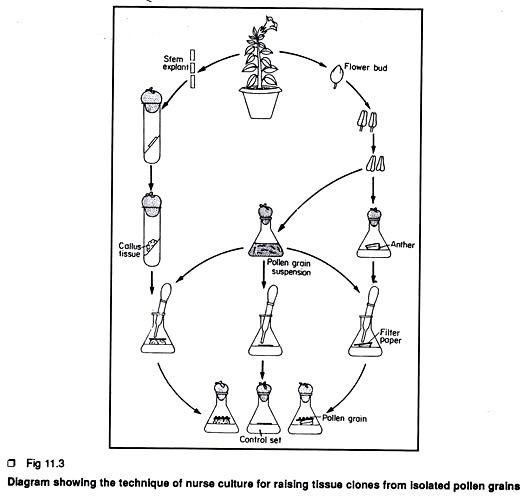
This method is known as nurse culture technique. Sharp et al. (1972) first introduced this method.
The steps are given below:
Step 1.
Selection of flower bud, sterilization, excision of anther, isolation of suitable pollen are the same as described previously.
ADVERTISEMENTS:
Step 2.
In this method, the intact anthers are placed horizontally on the top of solid or semisolid basal medium within a conical flask.
Step 3.
A small filter paper disc is placed over the intact anther and about 10 pollen grains in suspension are then placed on the filter paper disc. Hence the intact anthers are considered as the nurse tissue. A control set is also prepared in exactly the same way except that the pollen grains on filter paper are directly kept on solid medium. Sometimes, callus tissue derived from any part of the plant is used as nurse tissue (Fig 11.3).
Step 4.
ADVERTISEMENTS:
With this method, pollen grains in the control set did not grow at all. The pollen grains kept on nurse tissue grow and form a culture of green parenchymatous tissue in two weeks. Such tissue ultimately form the haploid callus tissue.