ADVERTISEMENTS:
The below mentioned article provides an outline on the techniques used in plant tissue culture.
Some of the techniques are: (1) Preparation of Culture Medium (2) Sterilization Procedure (3) Preparation of Aseptic Plants (4) Aseptic Techniques and (5) Incubation of Culture.
Several techniques have been adopted for in vitro plant cell, tissue and organ culture. Among them some are general techniques that are essentially followed in all experiments.
General Techniques:
Preparations of nutrient medium, sterilization, aseptic manipulation, maintenance of culture are the general techniques.
Technique # 1. Preparation of Culture Medium:
ADVERTISEMENTS:
Principle:
In vivo plant cells, tissues and organs get their appropriate nutrient and growth requirements from the intact plant body for their organised growth and development. Isolated cell, tissues and organs also need nutrients for their in vitro growth and development. So, nutrients are supplied artificially according to the medium formulated by several workers. The main objective of medium preparation is to culture the cell, tissue and organ in vitro.
Procedure:
ADVERTISEMENTS:
Media should be prepared with care and the following procedure is recommended.
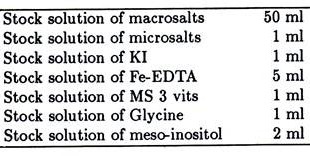
To make 1litre of MS medium:
(i) Dissolve 30gms cane sugar in 200 ml DDH2O. Mix 1-2gms activated charcoal and filter through filter paper, set inside the Buchner funnel fitted on a special conical flask with small side arm attachment. Filtering is done by using a suction pump.
(ii) Take DDH2O in another flask and add in sequence the appropriate amount of stock solution as follows—
Desired concentrations of auxin and/or cytokinin are added from stock solution according to the formula:
Desired concentration/Stock concentration
= amount (ml) of stock solution to be taken for one litre medium.
If the quantity of the medium is less than one litre, then hormones are added using another formula—
ADVERTISEMENTS:
Required concentration X Volume of medium/Stock concentration X1, 000
= amount (ml) of stock solution to be added.
(iii) Pour filtered sucrose solution and salt, vitamins, amino acid, hormone solution mixture into a one litre measuring cylinder. Make the final volume to one litre with DDH2O. Shake well to mix up uniformly.
(iv) Adjust the pH of the liquid medium 5.6-5.8 with the aid of 0.1(N) HCl or 0.1(N) NaOH. This operation is done using the pH metre.
ADVERTISEMENTS:
(v) Add 5% to 8% agar to the liquid medium to make solid medium. Heat to 60°C to dissolve the agar completely. Otherwise, without adding agar, liquid medium can be used for culture.
(vi) Dispense the culture medium into culture tube (20 ml/tube) or wide mouth conical flask (25-40 ml/flask). Insert non-absorbent cotton plug wrapped with gauge cloth. Cover the plug with the help of brown paper and rubber band.
(vii) Medium is finally sterilized by autoclaving.
Technique # 2. Sterilization Procedure:
Principle:
ADVERTISEMENTS:
The culture medium, especially when it contains sugar, will also support the growth of micro-organisms like bacteria, fungi etc. So if they come in contact with medium either in cellular form or in spore form, the micro-organisms grow faster than the higher plant tissue due to their brief life cycle and will kill the tissue. The micro-organisms may come from glass vials, instruments, nutrient medium used for culture and even from plant material itself. Therefore, the surface of plant tissue and all non-living articles including nutrient medium should be sterilized.
Procedure:
(i) Sterilization of non-living Articles:
The routine sterilization procedure of non-living articles such as nutrient medium, glass goods, distilled water, instruments (wrapped with brown paper) is by autoclaving under steam at a pressure of 15 lb/in2 and a temperature of 120°C for 15 minutes.
ADVERTISEMENTS:
Thermolabile compounds are often added in the medium and such medium is sterilized either at room temperature or in cold by passing through bacterial filter.
An alternative method of sterilizing glass goods and instruments is by heating in an oven at 150°C for 3-4 hrs.
It should be noted that when autoclaving screw capped glass vials, care should be taken to ensure that the caps are not closed too tightly so that gases can expand without the risk of explosion.
(ii) Sterilization of Plant Material:
Plant material which is to be cultured, should be surface sterilized to remove the surface borne microorganisms. This procedure is done in front of a laminar air flow or inside the inoculation chamber before the plant material is inoculated onto the culture medium (Fig 1.10).
(1) Thoroughly washed plant material or ex- plant in tap water is immersed in 5% v/v solution of liquid detergent such as ‘Teepol’ for 10-15 minutes. Then wash the material thoroughly in tap water and finally in distilled water. This step can be done in the general laboratory. Subsequent steps are done in front of a laminar air flow or the pre-sterilized inoculation chamber.
ADVERTISEMENTS:
(2) Dip the explants in 70% ethyl alcohol for 60 seconds.
(3) Immediately transfer the material into an autoclaved jaw bottle and pour 0.1% mercuric chloride (HgCl2) 5-10% Sodium hypochlorite (v/v) solution. Keep them for 10- 15 minutes. During that period, the bottle is frequently swirled for shaking so that all surfaces of plant material come equally in contact with sterilant.
(4) After 10-15 minutes, decant the sterilant and wash the explants thoroughly with several changes of autoclaved distilled water to remove all traces of sterilant.
(5) Then the explants are ready for culture.
Technique # 3. Preparation of Aseptic Plants:
Principle:
ADVERTISEMENTS:
Surface sterilization of plant tissue may cause some deleterious effect because most of the sterilants are toxic chemicals. Seeds can more or less resist such deleterious effect due to the presence of its seed coat. So to avoid the surface sterilization of plant tissue, seeds are surface sterilized and are cultured on simple basal nutrient medium.
Seeds in culture germinate and give rise to an aseptic seedling. Explants from such seedlings grown under aseptic and controlled conditions are the most suitable material for culture and need no further surface sterilization.
Procedure:
(1) Wash the dry seeds thoroughly with tap water (Fig 1.11).
(2) Dip the seeds in 5% Teepol solution (v/v) for 10-15 minutes. Decant the Teepol solution and wash the seeds again with tap water and finally with distilled water.
(3) Rinse the seeds with 70% ethyl alcohol for 1 minute.
(4) In front of laminar air flow, transfer the seeds into an autoclaved bottle and pour 0.1% HgCl2 solution (w/v) so that seeds are immersed. Leave for 10-15 minutes. Stir the bottle frequently.
(5) Decant the sterilant and wash 3-4 times with autoclaved distilled water.
(6) Transfer the seeds from bottle to autoclaved petridish with the aid of sterile forceps.
(7) Open the closure of the culture vial containing the basal nutrient medium. Flame the neck of the culture vial and in quick succession transfers a few seeds on to the medium. Replace the closure.
(8) Incubate the seeds in continuous dark either at room temperature or at 25-28° C.
Technique # 4. Aseptic Techniques:
Principle:
Precautions must be taken to prevent the entry of any microorganism at the time of transferring the surface sterilized explants on the nutrient medium (inoculation) using the sterilized instruments. For this reason, manipulation and transfer should ideally be carried out under aseptic condition. Starting from surface sterilization to inoculation, all operations should be done aseptically.
Procedure:
A typical procedure of aseptic technique is given below:
(1) Put all the sterilized articles (media, instruments, glass goods etc.) for inoculation on the glass racks of the inoculation chamber. Alternatively, if laminar air flow is available, keep all articles on the table of air flow cabinet. Laminar air flow blows bacteria- free air over the working surface.
(2) Put on the switch of UV lamps of inoculation chamber for one hour before work. In case of laminar air flow, the power switch is put on and allows the air flow to blow air for at least 15 minutes before work.
(3) Put off the UV lamp before entering inside the inoculation chamber. Do not put off laminar air flow. The working glass table top of the inoculation chamber or the table of laminar air flow is swabbed with alcohol before starting work.
(4) Wear a clean apron and use a mask. Clean the hands with alcohol and dry it.
ADVERTISEMENTS:
(5) Pour alcohol in a clean coupling jar and dip all instruments into it. Light the spirit lamp. Take the surface sterilized or aseptic plant material in a, sterile petri dish.
(6) Flame the neck of culture tube or flask and in quick succession remove the plug of glass vials. Transfer the tissue onto the medium and replace the closure. Each time, the instruments are passed through the flame of the spirit lamp.
Precautions:
(1) Always keep away the hands moistened with alcohol from the spirit lamp. So dry the alcohol first.
(2) Exposure to UV light builds up a high concentration of Ozone gas (toxic) inside the closed chamber. It is, therefore, healthy to enter the chamber only 15-30 minutes after switching off the UV lamp.
(3) Do not dip hot instruments in alcohol and don’t use hot instrument for cutting or holding the plant material.
(4) Work carefully and try to ensure that media and plant tissues are exposed for the plant material.
(5) Don’t heat the neck of the glass vials excessively.
Technique # 5. Incubation of Culture:
Principle:
High temperatures are likely to lead to dissociation of the culture medium and tissue damage while at very low temperatures tissue growth is slow. Again some tissues grow well in dark while others need both light and dark conditions. Low humidity causes the quick desiccation of culture medium and high humidity is favourable for the contamination of culture medium. Therefore, cultures are incubated in a culture room where light, temperature and humidity are controlled.
Procedure:
(1) After inoculating the tissue onto the culture medium, cultures are incubated on culture rack at 25-28°C constant temperature.
(2) Culture tubes are placed at 30-45° inclined position. For this purpose a long wooden stick or an empty paper cover of fluorescence lamp is placed on the middle of culture rack and lay the plugged end of the culture tube on the support.
(3) Illumination is provided by cool-white fluorescent light placed about 18 inches above the culture to give a light intensity of 4 – 10 x 103 lux for 16 hours.
(4) If light is not necessary, then put off the light and cover the whole rack with a black cloth.