ADVERTISEMENTS:
Here is a list of top thirty five interview questions on Plant Physiology which will help you to succeed in an interview.
Q.1. What is meant by Plant Physiology ?
Ans. Plant physiology is a basic discipline which intimately interfaces with biochemistry, agronomy, genetics and plant breeding, soil science, plant pathology, microbiology and other related areas. Plant physiology explains various aspects of plant growth. It tells us how the constituent cells which may look alike individually, respond to a division of labour and perform organ-specific functions.
Plant physiology also tells us how plants respond to the changing environments to which their cells are so freely exposed and control their behaviour in growth, senescence and rest.
ADVERTISEMENTS:
The gain in knowledge in this discipline has become really enormous. In the last few decades, several discoveries have been made which have revolutionized the basic concepts of plant physiology and have added to our knowledge of this subject.
Many older hypotheses and assumptions are now invalidated, yielding place to new ideas. However, many important new discoveries are not entirely new but are additions to already existing concepts.
The rapid expansion of knowledge about plant processes has resulted more from continuous improvements in experimental techniques and instruments than from recognition of new concepts.
Q.2. Define the term Atom ?
Ans. The term ‘atom’ comes from two Greek words meaning ‘not’ and ‘cut’. It is the smallest portion of an element which cannot be cut into still smaller portions. All material bodies, whether elements or compounds, are fundamentally granular in structure, i.e., made up of discrete particles separated by interspaces.
ADVERTISEMENTS:
These ultimate constituents of matter are called atoms. They can take part in chemical reactions. This was the ancient concept of atomism of John Dalton (1808). Modern scientific researchers have, however, decided in favour of the atomic theory of matter, though the ancient concept of atoms as the ultimate and indivisible units of matter has been modified.
The concept of atomism is supported by the following facts:
(i) Compressibility of matter
(ii) The phenomena of diffusion, osmosis, Brownian movement, solubility
(iii) The regular forms of crystals governed by definite simple laws
(iv) The law of multiple proportions
(v) The periodic law among elements.
Q.3. What do you mean by Thermodynamics ?
Ans. Thermodynamics (Greek: therme, heat and dynamis, power) is generally defined as the study of the relationship of heat to mechanical and other forms of energy. It is more justifiable to define the term as the study of the relationships among various forms of energy. Energy conversions are continually happening around us. If a light switch is put on, the electrical energy is converted into light and heat energy.
A fire fly converts the chemical energy from its foods into light energy and motion energy. When something heavy is lifted, chemical energy in the muscles is converted into the potential energy of raised object. The more work is done the more energy is converted. The energy chain can be explained as such.
ADVERTISEMENTS:
Inside the sun, nuclear energy is converted to heat and light energy. The green plants convert light energy from the sun into the chemical energy of sugar by the process of photosynthesis.
If anybody eats green plant, the chemical energy it contains is transferred to his body. It is used for the activities, such as breathing and moving. Winding up an alarm clock changes this chemical energy to elastic potential energy in the spring.
The potential energy of the wound spring is converted to movement energy of its hands and sound energy of its ticks. The clock keeps working until the spring is unwound and has lost its potential energy. Lord Kelvin (1824 – 1907) helped to find the science of thermodynamics, establishing clear relationships between heat, work and energy.
Life obeys the laws of thermodynamics, although living systems present some practical challenges to thermodynamic analysis. Classical or equilibrium thermodynamics investigates the feasibility and extent of chemical reactions by measuring properties of matter in bulk.
Q.4. How do you define the term Free Radicals ?
ADVERTISEMENTS:
Ans. Free radicals are atoms or molecules having an unpaired electron and are produced when bonds are broken symmetrically in hemolytic reactions. In such reactions electron pairs are divided, and each nucleus receives one electron. A free radical containing one or two unpaired electron(s) is (are) called monoradicalor biradical respectively. Free radicals are unstable owing to the presence of unpaired electrons.
The triphenylmethyl radical (C6H5)3C is rather stable, because the unpaired electron is not localized at the central carbon atom, and its density is distributed between the central carbon atom and all the carbon atoms of the three benzene rings (i.e., between 19 carbon atoms) owing to conjugation.
The free radical can become stable also when reagent molecules cannot easily enter the region where the unpaired electron is localized (“screening”). If both stabilizing factors, conjugation and screening, act simultaneously, free radicals can become very stable and act as ordinary organic substances in chemical reactions.
Short-lived free radicals (half-life of H3C is 0.005 s) can be formed as intermediate reaction products, and they can affect the course of a reaction owing to their high reactivity. Many practically very important reactions occur with the participation of free radicals, e.g., reactions of paraffin halogenation, thermal decomposition of hydrocarbons, oxidation, and polymerization.
ADVERTISEMENTS:
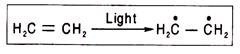
Semiquinones in the respiratory chain are free radicals as they have an unpaired electron. They are capable of accepting and donating electron(s). A free radical is almost always produced when a double bond between two carbon atoms is changed to a single bond.
In free radicals the spin of an unpaired electron is not compensated for by a partner’s electron spin in the opposite direction and so the resulting spin is either + ½ or – ½. In a biradical the resulting spin is either + I or – I. As resulting spin of a free radical is other than zero, it acts as a paramagnetic substance. For these properties the free radicals are very useful in detecting photo biological processes.
Q.5. What do you mean by Primordial Biomolecules ?
Ans. A. I. Oparin in the Soviet Union and J. B. S. Haldane in Britain during the 1920s first postulated that organic compounds arose by reactions between various inorganic components of the atmosphere and geosphere, activated by the energy of UV-light, electric discharges, heat, or other forms of energy.
ADVERTISEMENTS:
These organic compounds were mainly amino acids and sugars, nitrogenous bases, etc., which gradually concentrated in the primitive sea. These organic compounds are known as the primordial biomolecules.
In the later period of chemical evolution of earth these primordial building blocks are believed to have undergone abiotic condensation to form primitive polypeptides, polynucleotides, polysaccharides and lipids.
From this primordial organic soup, the first living organisms are believed to have arisen. Such compounds or their precursors have been detected in ancient fossils, in meteorites and in interstellar space. But a big gap remains between these molecules in a state of solution and an organized cell.
The abiotic formation of primordial biomolecules may be understood but their transformation to the cellular organization when life first appeared is indeed a puzzling phenomenon. It is highly speculative. Formation of proteinoid droplets having a membrane like structure has been suggested by Sidney Fox at the University of Chicago and is very well documented in the laboratory.
Q.6. Define Nucleic Acids ?
Ans. The nucleic acids are of fundamental importance to living organisms as they control all the cellular activities directly or indirectly through the synthesis of all cellular proteins, and hence of all cellular constituents. Both types of nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are normally present in the nucleus of a cell. RNA is chiefly located in the cytoplasm outside the nucleus.
Nucleic acids like proteins are macromolecules built from three types of repeating subunits:
ADVERTISEMENTS:
(i) Nitrogenous bases — purines and pyrimidines,
(ii) Pentose sugars, and
(iii) Phosphate units.
The nucleic acids are strongly acidic and at physiological pH carry a high density of negative charge. For that reason they usually remain associated with different types of cations like Mg2 + and basic proteins like histones. DNA was discovered by a Swiss physician F. Miescher in 1869. It is the genetic material in most organisms and carries the hereditary informations.
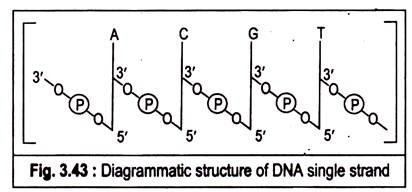
Q.7. Explain molecular structure of DNA ?
Ans. Polymers of deoxyribonucleotides are known as deoxyribonucleic acids (DNA). The constant feature throughtout the molecule of DNA is its backbone structure consisting of deoxyriboses linked by phosphodiester bridges. The 3′-hydroxyl group of the sugar moiety of one deoxyribonculeotide is joined to the 5′-hydroxyI of the adjacent sugar by a phosphodiester linkage.
The variable part of the DNA structure is the base, the permutation and combination of which results in the formation of different species of DNA.
ADVERTISEMENTS:
The structure of DNA can be concisely represented in the following figure:
The DNA molecule is formed by the end to end joining of a large number of nucleotides forming two long anti-parallel polynucleotide chains running in opposite directions forming a double helix around a central axis. The successive nucleotides of DNA are covalently linked to each other through phosphate group bridges.
Thus the covalent backbones of nucleic acids consist of alternating phosphates and pentose groups, whereas the characteristic bases may be regarded as side groups joined to the backbone at regular intervals. The backbones are highly polar, since the phosphate groups are acidic and have negative charges at the cell’s pH.
On the other hand, the purine and pyrimydine bases which are relatively insoluble in water are hydrophobic. The DNA strands have a specific polarity or direction because all the inter-nucleotide phosphodiester linkages have the same orientation along the chain. Because of this polarity each linear nucleic acid strand has a 5′ and a 3′ end.
Q.8. What is the weight of E. coli DNA ?
Ans. DNA molecules are the largest macromolecules, which are very difficult to isolate without fragmentation. The E. coli DNA has a molecular weight of 2.6 x 109. The molecular weights of viral DNAs range from 1 to 350 x 106. The molecular weight of a single nucleotide is 300 to 350. Therefore, there are about 3,000 nucleotides per million molecular weight of DNA.
Q.9. How to measure the length of DNA ?
Ans. The quantity of DNA is usually measured by picogram (1 pg = 10-12g). One picogram amount of DNA contains about a stretch of 31 cm DNA double helix. So, if the weight of the DNA present in the nucleus can be measured, its length can be calculated easily.
It has been estimated that each human diploid cell contains 5.6 pg of DNA, which is equivalent to 173.6 cm in length (Dupraw and Bahr, 1968). Likewise, a diploid cell of Trillium contains 37.2 m double stranded DNA equivalent to 120 pg by weight. The polytene chromosome of Drosophila contains 293 pg of DNA, which is equivalent to 90.83 m in length.
Q.10. What are the functions of Repetitive DNA ?
Ans. The function of highly repetitive DNA, most of which is located in genetically inactive heterochromatin regions of chromosome is completely unknown.
Postulated function of highly repetitive DNA includes:
(i) Structural or organizational role in chromosomes.
(ii) Involvement in chromosome pairing during meiosis.
(iii) Involvement in crossing-over or recombination.
(iv) Protection of important structural genes.
(v) A repository of unessential sequences for the use in the future evolution of the species.
(vi) No function at all — just “junk” DNA or “selfish” DNA or “molecular parasite” which is carried along by the process of replication and segregation of chromosomes.
The validity of any of these postulations remains to be established.
Q.11. How Restriction Maps are useful ?
Ans. Restriction maps are useful laboratory tools as restriction sites are physical reference points on a DNA molecule. These are therefore a convenient framework for locating particular base sequences on a chromosome and for comparing different chromosomes. Individuality of an organism is derived from their high degree of genetic polymorphism.
Homologous chromosomes differ in sequence, on an average, every 200 to 500 bp. These differences create or eliminate restriction sites. The homologous chromosomes after restriction enzyme treatment therefore contain fragments with different lengths showing restriction fragment length polymorphisms (RFLPs).
The two homologous segments in the Fig. 3.52 differ in the number of restriction sites. An individual with one of each homologue would yield fragments A, B, and C. RFLPs are particularly valuable for diagnosing inherited diseases for which the molecular defect is unknown.
Q.12. What are the general functions of Mineral Elements ?
Ans. Essential elements perform the following in plant life:
(i) The essential elements serve as the framework elements or building materials for protoplasm, cell wall, enzymes and so on.
(ii) Osmotic pressure in plant cells is developed by essential elements.
(iii) Though inorganic constituents have little influence on pH, certain ions like phosphate, bicarbonate and carbonate may act as buffers and thus resist marked change in pH. Plant tissues usually control the degree of acidity and buffer action primarily by organic acids.
(iv) Desirable degree of hydration of cell colloids is maintained by the essential elements. In general, monovalent cations increase hydration whereas divalent, particularly polyvalent cations decrease it.
(v) Permeability characteristics of membranes is regulated by the essential elements. It is influenced by cations and anions in the medium with which the membrane is in contact. Some ions have decreasing effect on the permeability while others have an increasing effect.
(vi) Essential elements show antagonistic effect. Antagonism pertains to those interactions in which the normal effect of one ion is counteracted or neglected by that of another ion.
(vii) Elements like iron, copper, zinc, manganese, etc., act as catalysts in various enzymatic reactions in plants.
Q.13. Explain the mechanism of Ion Absorption in plant ?
Ans. The mineral salts remain in the soil solution in dissociated condition. The essential ions are absorbed in different amounts by the root hairs and are then trans-located through the xylem stream to the different parts of the plant body.
Previously, it was assumed by plant physiologists that inorganic salts were passively absorbed en mass along with water. But at present it is consistent with the notion that ions are absorbed by different mechanisms. Time to time different theories have been proposed by different workers to explain the mechanism of ion absorption.
Early workers continuously produced physical mechanisms and models to explain salt absorption. Finally, it has been shown that salt absorption is largely dependent on metabolic energy, i.e., the uptake of salt is predominantly active.
There are two major mechanisms for ion uptake — non-mediated uptake and mediated uptake. An ion, like any other body, will move in a given direction only if driven by some force.
If ion transport in and out of cells occurs spontaneously down a gradient of electrochemical potential energy, it is called passive uptake and when ion driven up such a gradient by some process directly coupled to metabolism, it is called metabolic or active uptake. Non-mediated transport is always passive whereas mediated transport may be either passive or active.
Q.14. What do you mean by Ascent of Sap ?
Ans. Movement of the absorbed water through the vascular system from the xylem terminals in the root to those in the leaf, is called ascent of sap. Sometimes it covers a distance of more than 111 meters against gravitational pull as in the case of a Californian Sequoia sempervirens (111.6 m or 366.2 ft.) and an Australian Eucalyptus regansi 114.4 m).
The problem for plant physiologists is, the mechanism of this movement. One atmospheric pressure supports a column of water 10.3 m or 34 ft. or a column of mercury 760 mm high. To raise water from the ground level to the top of the tallest tree requires a top to bottom pressure difference of about 22 atm or bars or 2.2 MPa (Mega Pascal). It is evident that water is not pushed to the top of tall trees by atmospheric pressure.
Q.15. Explain the concept of Path of Water ?
Ans. It is well established that longitudinal water movement through the plant takes place predominantly through the xylem, but the whole cross-sectional area of the root or stem may be available for flow, except in dense woody regions. Evidence for the xylem path comes from the observation that water flow is not immediately stopped when a ring of tissues external to the xylem is removed.
If a section of xylem is removed leaving other tissues intact, ascent of sap is impaired. When solutions labeled with water-soluble dyes, radioactive solutes or water containing 3H or 18O are supplied to plants, the label is rapidly detected in the xylem of roots and stems, particularly in the vessels and tracheid’s.
If the cut end of a flowering twig of tuberose is dipped in eosin solution its flowers turn red. The t.s. of the stem shows only xylem vessels stained red. That the lumen of xylem vessels are the path through which water moves can be shown by clogging the vessels of a balsam twig by gelatin jelly or low melting point paraffin.
The cut end of the twig is dipped in molten gelatin jelly or low melting paraffin. After a few minutes the gelatin or paraffin enters the xylem cavities and block them. A section is removed from the cut end to expose the vessel walls and xylem parenchyma, although the cavities are still blocked. Then the cut end is put in water.
The leaves on the twig are found wilting as water cannot go up through the wall of xylem elements sufficiently to maintain turgor pressure. This clearly indicates that water translocation takes place mainly through the xylem vessels.
Q.16. What is the meaning of CAM ?
Ans. Crassulacean acid metabolism (CAM) is a specialized photosynthetic process which is characterized by the following criteria as given by Osmond (1978) and Kluge and Ting (1978).
These are:
(i) CO2 uptake takes place mainly at night,
(ii) Stomata are open at night and are usually closed during the day when CO2 uptake is almost negligible,
(iii) Malate accumulates at night by carboxylation of PEP by PEPcase,
(iv) Decarboxylation of malate during the day yields CO2 inside the photosynthetic tissue which is fixed normally by RuBPcase in the C3 cycle, thus permitting CO2 assimilation without CO2 entry directly from air.
CAM has been named after the family Crassulaceae in which the phenomenon was first observed. This process is also common in other families of higher plants including Cactaceae, Euphorbiaceous, Aizoaceae, Liliaceae, Bromeliaceous and Orchidaceae. A few economically important plants, including pineapple are CAM plants.
Q.17. State the significance of CAM ?
Ans. CAM is an adaptation to extremely xerophytic environments. Rates of carbon fixation in CAM, are, however, lower than those of C3 and C4. Under conditions of severe water stress, C3 photosynthesis may cease altogether whereas CO2 uptake continues in CAM plants.
Under severe drought condition, net carbon loss occurs in C3 and C4 leaves due to dark respiration, whereas CAM tissues may eliminate respiratory loss of CO2 since these are able to retain and re-fix the respired CO2. This situation is clearly demonstrated in certain cacti which can survive in extremely hot regions.
The stomata remain closed not only during the daytime but also may be closed during the night. Under this condition, evaporative water-loss as well as CO2 exchange is almost nil. Thus there is no growth but the plant can survive by fixing CO2 made available internally by photorespiration and dark respiration.
The CO2 and PEP resulting from the normal respiratory process may be converted back to malate which upon decarboxylation provides CO2. This recycling of CO2 through CAM pathway is referred to as ‘idling’ CAM which may undergo transition to productive CAM when water supply is not limiting. In this way, these cacti can maintain almost constant dry weight over a long period of drought condition.
There may be two types of CAM plants. In one type represented by the cacti such as Opuntia basilaris and Zygocactus truncates which are obligate CAM, the plants behave like CAM even during periods of abundant water supply.
By contrast, some CAM plants are facultative or inducible, such as Mesembryanthemum crystallinum, Kalanchoe tubiflora and a few others, which shift from CAM to C3 photosynthesis when adequate water is available. Such facultative CAM species require inorganic salts, particularly NaCl, in the soil for the development of CAM character which is similar to the sodium requirement of C4 plants.
Q.18. What do you mean by Gluconeogenesis ?
Ans. Under either aerobic or anaerobic conditions, the central pathway of carbohydrate breakdown involves the conversion of glucose-6-phosphate to pyruvate, catalysed by the enzymes of glycolysis.
In contrast, gluconeogenesis means the synthesis of new glucose, which takes place in many different organisms by utilizing the central pathway of glucose catabolism in the reverse direction, i.e., the pathway leading from pyruvate to glucose-6-phosphate and ultimately to glucose.
In such central pathway of glucose biosynthesis, conversion of other simple precursors like lactate, certain amino acids into glucose is also possible. In photosynthetic organisms, however, biosynthesis of carbohydrates takes place by the most prominent processes utilizing solar energy, in which hexoses generated from carbon dioxide and water are converted into starch, sucrose, cellulose and other polysaccharides.
Q.19. State the role of Pentose Phosphate Pathway ?
Ans. In plant cells, the major part of glucose is subject to aerobic degradation in the consecutive processes of glycolysis, citric acid cycle and respiratory electron transport chain. In the course of these processes, ATP is synthesized by substrate-level phosphorylation and oxidative phosphorylation. The end products are water and carbon dioxide.
The pentose pathway is an alternative route for the oxidation of glucose. A number of different names have been assigned to the reaction sequence involved. Since the pathway diverges from glycolysis at the glucose-6-P level, it has been named the hexose monophosphate shunt.
Since pentose phosphates play an important role in the cyclic reaction sequence, it is referred to as the pentose phosphate cycle and since phosphogluconate is a key intermediate, the term phosphogluconate pathway is often used.
Here glucose is directly oxidized by dehydrogenases (i.e., first two reactions of the sequence), it is frequently referred to as the direct oxidation pathway. The term Warburg-Dickens-Horecker pathway is also used after the biochemists who were the principal investigators.
Q.20. What are the main functions of Pentose Phosphate Pathway ?
Ans. (i) To generate NADPH in cytosol for use in biosynthetic reactions
(ii) To provide ribose-5-P for nucleotide synthesis and
(iii) Erythrose-4-P for shikimic acid synthesis.
Q.21. Discuss the significance of Pentose Phosphate Pathway ?
Ans. The pentose phosphate pathway differs from glycolysis since in the initial oxidation reactions, NADP+ rather than NAD+ is used. Pentose phosphate pathway is characterized by CO2 production, while CO2 is not produced at all in the glycolytic pathway. ATP is not generated by re-oxidation of NADPH + H+.
Most of the NADPH produced can provide the reducing power for the synthesis of a series of compounds in cytosol like fatty acids, mevalonic acid and steroids. Conversion of pyruvate to oxaloacetate by malic enzyme may occur in the presence of these reducing equivalents.
Moreover, the cycle provides pentose phosphates which may be utilized as precursors for the synthesis of nucleotides and nucleic acids. Ribose-5-P, an intermediate of the pathway, reacts with ATP to form phosphoribosyl pyrophosphate (PRPP) which is used in nucleotide biosynthesis.
If necessary, intermediate like glyceraldehyde-3-P and fructose-6-P may enter the process of glycolysis and are thereby aerobically degraded. Thus, the cycle provides a storehouse for the building elements of different areas of metabolism.
Q.22. How to calculate the Standard Free Energy Change ?
Ans. The standard free energy change can be calculated from sample data on the enzyme malate dehydrogenase which catalyses the oxidation of malate to oxaloacetate
We are required to calculate the standard free energy change at 25°C at pH 7.0 of the above reaction at equilibrium when malate is oxidized to oxaloacetate, assuming malate = 10-3 M, oxaloacetate = 10-1 M. We can calculate the equilibrium constant from these values,
From this value of K’eq, the standard free energy change ΔG°’ is calculated from the equation (vi)
= – 1363 x log 100
= – 1363 x 2 = – 2726 cal mole-1
or – 2.726 kcal mole-1
Since the sign of the standard free energy change of the above reaction is negative, the conversion of malate to oxaloacetate is an exergonic process.
Q.23. Carbon and nitrogen exchange between the heterocyst and vegetative cells: Discuss ?
Ans. When cyanobacteria are deprived of an external source of combined nitrogen such as nitrate or ammonium, some of the vegetative cells of the filament develop into specialized, thick-walled cells called heterocyst’s. This complex cytodifferentiation event involves the cessation of transcription of vegetative cell genes and the initiation of new transcripts from heterocyst-specific genes.
As a result both the structure and enzymatic composition of the cell change over a period of 20-30 ours, which can no longer participate in cell division and develops a heavy investment around it. The prime function of the heterocyst is to harbour nitrogenase in an anaerobic environment.
The thick wall consists of three layers that prevent the entry of oxygen. The wall is more impermeable to oxygen than to nitrogen. The reaction for that is unknown. In addition the ends of heterocyst have polar nodules that prevents the entry of oxygen.
Micro-plasmodesmatal connections are there with the adjacent vegetative cells for the entry of nitrogen and other necessary metabolites while preventing the entry of oxygen.
Photosystem-I remains active in the heterocyst to provide ATP for N2-fixation through cyclic photophosphorylation. However, Photosystem-ll is inactive in the heterocyst and it lacks Rubisco. The primary source of reducing power must, therefore, be imported into the heterocyst from the vegetative cells.
A disaccharide, possibly maltose, is imported, hydrolysed and the product glucose is used to provide NADPH by oxidation via the first two enzymatic steps of the pentose phosphate pathway.
In exchange the fixed nitrogen is exported. Glutamine synthetase is responsible for the assimilation of ammonium. Glutamine is exported to the vegetative cells and converted to glutamate there by glutamate oxoglutarate aminotransferase (GOGAT).
Unlike their heterocystous relatives, the non-heterocystous cyanobacteria do not usually effect a spatial separation between the oxygen-sensitive process of nitrogen fixation and photosynthetic oxygen evolution.
This lack of spatial separation between the two processes indicates that light generated ATP and NADPH are utilized in N2-fixation by non-heterocystous cyanobacteria. But there is no direct evidence that photosynthesis directly provides reducing power for nitrogen fixation.
Trichodesmium, a marine filamentous form, fix nitrogen mainly in the dark. Under these conditions photosynthesis supports nitrogen fixation only indirectly through accumulation in the light of carbon reserves that are broken down to support nitrogen fixation in the dark.
Q.24. Define Lipids ?
Ans. The lipids are a heterogenous group of compounds made up of various groups like neutral fats, waxes, phospholipids, sphingolipids and related compounds.
A common property characteristic of lipids is:
(i) Insolubility in water
(ii) Solubility in non-polar organic solvents like ether, chloroform and benzene.
Q.25. What are the various types of Lipids ?
Ans. A. Simple Lipids:
These are esters of fatty acids with various alcohols.
These are subdivided into:
(i) Fats:
These are esters of fatty acids with glycerol, a trihydric alcohol. Fats are solid at room temperature, while a fat in liquid state is known as an oil.
(a) Neutral Fats:
Neutral fats are the esters of the trihydric alcohol, glycerol with fatty acids which are either saturated or unsaturated, un-branched with usually an even-number of carbon atoms. If only one of the hydroxyl groups in the glycerol molecule is esterified with a fatty acid, the compound is a monoglyceride or may also be termed as monoacyl glycerol.
If two or all three hydroxyl groups are esterified, the resulting compounds will be a diglyceride (diacylglycerol) or triglyceride (triacylglycerol). In the oil and fatty seeds of higher plants, the triglycerides are the major lipids.
Fats and oils are distinguished from each other by their physical state at room temperature, fats being solids and the liquid being oils. Fats contain saturated fatty acids in their structures whereas the oils contain mainly unsaturated fatty acids.
The name of the neutral fat is derived from the names of the constituent fatty acids. Thus, tripalmitin and triolein (liquid) contain three palmitic acid and three oleic acid residues per molecule, respectively.
More than one type of fatty acid may be linked to the glycerol through esterification. Thus, oleodipalmitin contains one residue of oleic acid and two of patmitic acid whereas oleodistearin has one oleic acid and two stearic acid residues.
In alkaline condition, triglycerides are degraded to free glycerol and alkali salts of the fatty acids which are soaps. This process which is used in the production of soaps is termed saponification which is the hydrolytic cleavage of the triglycerides. Lipid molecules may also undergo enzymatic hydrolysis under the influence of lipase and the products are glycerol and fatty acids.
(ii) Waxes:
These are esters of fatty acids with monohydric alcohols having higher molecular weight.
B. Complex Lipids:
These are esters of fatty acids with alcohol having additional groups.
Complex lipids can be further subdivided into:
(i) Phospholipids:
Such lipids contain in addition to fatty acid and an alcohol, a phosphoric acid residue. They may also contain nitrogenous bases and other substituents.
These are subdivided into:
(a) Glycerophospholipids— here the alcohol is glycerol,
(b) Sphingophospholipids— here the alcohol is sphingosine.
(ii) Glycolipids:
These lipids contain carbohydrate in addition to fatty acids and alcohol.
(iii) Other Complex Lipids:
Sulpholipids, amino lipids and lipoproteins ma be included in this category.
Q.26. State the functions of Lipids ?
Ans. Fat as a storage material in plant cells and tissues serves to fulfil two functions:
(i) As substrate in the production of energy
(ii) As starting material in synthesis of cellular metabolites.
Q.27. Discuss the difference between Fatty Acid Synthesis and Fatty Acid Oxidation ?
Ans. Fatty Acid Synthesis:
1. Occurs in cytosol.
2. 2-C unit is added through malonyl-ACP (3-C) accompanied by CO2 liberation.
3. ACP is the acyl group carrier.
4. β-ketoacyl → β-hydroxyacyl reaction is NADP+ specific.
5. β-hydroxyacyl intermediate is D-isomer.
6. Crotonyl → butyryl step is NADP+ specific.
7. Acetyl CoA carboxylase being an allosteric protein responds to citrate and HCO3 as positive modifiers.
Fatty Acid Oxidation:
1. Occurs in mitochondria.
2. 2-C unit is removed in the form of acetyl CoA.
3. CoA is the acyl group carrier.
4. β-hydroxyacyl → β-ketoacyl reaction is NAD+ specific.
5. β-hydroxyacyl intermediate is L-isomer.
6. Butyryl → crotonyl step is FAD specific.
7. No such response.
Q.28. State the discovery of Auxins ?
Ans. As early as 1928, Went discovered that a substance diffused out of coleoptile tips onto agar blocks which then caused curvatures in the Avena test.
He assumed that this biological activity was due to only one substance coming from the coleoptile tips and this was auxin. A few years later, Kogl et al., reported the isolation of auxin a and auxin b from human urine which were believed to be cyclopentane derivatives.
Neither the existence not the proposed structures of these compounds could be subsequently confirmed. Another compound, at first called hetero-auxin (different auxin) was soon afterwards identified in urine, then from yeast and also from culture medium of Rhizopus suinus. This compound was found to be indole-3-acetic acid (IAA).
Subsequently, it was isolated from corn seeds. It was later identified by chromatographic, chemical, colorimetric and other methods in a wide variety of plants, and is recognized as the principal auxin of higher plants.
Q.29. State the discovery of Gibberellins ?
Ans. The story of the discovery of gibberellins actually began in the last decade of the nineteenth century, when a disease of the rice plants was being studied in Japan. When the rice plants are infected by the ascomycetous fungus, Gibberella fujikuroi (asexual or imperfect stage is Fusarium monoliforme), they become excessively tall and chlorotic with reduced root growth and tillering and eventually die.
The Japanese farmers called this the ‘bakanae’ (foolish seedling) disease of rice. In 1926, Kurosawa, a Japanese plant pathologist in Taiwan grew the causal fungus in nutrient medium and showed that the cell-free culture medium could itself induce the abnormal elongation of rice seedlings. Yabuta and Sumiki in 1938 isolated the active factor from Gibberella fujikuroi culture filtrates and assigned the name ‘gibberellin’ to it.
The initial research work on gibberellins was published in Japanese language and remained unknown to the western world for about 10 years because of the Second World War. In 1 954-55, American and British scientists like Stodola, Brian, Cross and others isolated and purified the gibberellins and named it gibberellic acid (GA3).
This was followed by intensive research in 1950s on the effects of gibberellic acid of fungal origin on higher plants. In 1956, West and Phinney in U.S.A. and Radley in England discovered that GAs occur naturally in higher plants. Thus, it became apparent that the plants possess a second group of growth regulators which play an important role in the control of growth and development.
As of 1990, 84 gibberellins have been discovered in fungi and higher plants. Of these, 73 occur in higher plants, 25 in Gibberella fungus, and 14 in both.
These are abbreviated as GA with a subscript such as GA1, GA2, GA3, and GA4 and so on of which GA3 is commonly known as gibberellic acid. Gibberellins and GA-like substances have been found in almost all the representatives of the plant kingdom starting from bacteria through fungi to angiosperms.
Besides the fungal sources of GAs, the entire vegetative organs of higher plants, particularly the shoot-tip portion, are the sites of GA synthesis. Fruits and seeds, particularly immature seeds contain an abundant amount of GAs, much higher than vegetative tissues.
Q.30. Describe the structure of Gibberellins ?
Ans. The gibberellins are a large family of diterpene acids. The systematic nomenclature is based or the gibbane ring system which is common to all known gibberellins. A more appropriate parent skeleton gibberellane has been proposed as the basic ring structure which has a numbering system corresponding to other cyclic diterpenes especially kaurene, a crucial intermediary in GA biogenesis.
Individual GAs are referred to as GAX in the series GA1 – GAn, the numbers usually follow the order of discovery. GAs can be subdivided as C20 and C19 compounds according to the number of carbon atoms. C20 – GAs have carboxyl groups in positions 7 and 18 and some have also in 20, while some have an aldehyde group in the latter position.
The C19 GAs are all monocarboxylic acids with carboxyl group in position seven and have lactone configuration in A ring which arises by the loss of carbon – 20.
Q.31. State the discovery of Cytokinins ?
Ans. In the 1950s, Folke Skoog and his associates were engaged in tissue culture experiments. While using coconut milk and yeast extract containing growth factor for growing tobacco tissues, they found that the active growth factor was a purine derivative. Since nucleic acids contain purines, C.O. Miller, one of Skoog’s associates used an old sample of Herring sperm DNA which was capable of causing tobacco cells to divide.
It was of interest to note that fresh samples of DNA failed to show any bioactivity, but when aged in an autoclave these became active. The conclusion is that the cell division factor is the breakdown product of nucleic acid which was later recognised to be a cytokinin.
Q.32. How Cytokinins occurred ?
Ans. Cytokinins are widespread in plants. Embryos, developing fruits and seeds, and young fruits are a source of cytokinins in plants. In an intact plant, roots are the organs where cytokinins are synthesized and are major sources of cytokinins. Since the cytokinin level in the xylene sap is quite high, the logical conclusion is that the cytokinin synthesized and supplied by roots can regulate developmental changes in the plant.
Q.33. State four bioassay methods of Cytokinins ?
Ans. The bioassay methods which may be employed for the estimation of cytokinin in a plant tissue may be summarized as follows:
(i) Cell Division Test:
The most satisfactory method of cytokinin bioassay is based on the induction of cell division in tissue cultures. Best tissues used for this bioassay are tobacco pith tissue and soybean callus tissue. Tissues are generally grown for 2-4 weeks on media in which cytokinins have been added, after which fresh and dry weights are determined and compared with suitable controls.
(ii) Chlorophyll Preservation Test:
This bioassay is based on the Richmond-Lang effect which suggests that cytokinins are involved in the retardation of senescence through the preservation of chlorophyll. In this bioassay, leaf discs are placed in test solutions in dark, then after a definite period chlorophyll is extracted and measured. A linear relationship is found between the amount of chlorophyll retained and log of cytokinin concentration.
(iii) Cell Enlargement Test:
Leaf discs of Raphanus sp. are floated on cytokinin solutions in light for a definite period of time and then weighed after blotting off the excess liquid. It is also suggested to measure the increase in diameter of the leaf discs caused by cell enlargement which is proportional to cytokinin concentrations.
(iv) Differentiation Test:
Generally, moss Protonema respond to cytokinin by an increase in bud formation and a bioassay seems to be possible by using this cytokinin effect.
Q.34. State the discovery of Abscisic Acid ?
Ans. The history of the discovery of abscisic acid (ABA) is interesting. In the 1960s, groups of workers were independently attempting to isolate and purify a growth-regulating substance.
One group, led by Addicott and his associates Ohkuma, Smith and Thiessen at the University of California, Davis, obtained from young cotton bolls two partially purified fractions which accelerated leaf abscission in young cotton seedlings and which they called abscisin I and abscisin II.
At about the same time, Rothwell and Wain (1964) at Wye College, London University were attempting to identify a substance which accelerated flower drop in lupins.
Further work, was however, concerned with the isolation and identification of abscisin II. Following a different approach, Wareing and his co-workers at Aberystwyth, Wales, obtained from sycamore (Acerpseudoplatanus) leaves an acidic extract which was highly active as a growth inhibitor and which was able to induce the formation of resting buds in sycamore seedlings when applied as solution to leaves.
The active substance was named dormin since it caused bud dormancy. At this stage, further purification of dormin was taken over by Cornforth at Milstead Laboratory of Shell Research Ltd, UK and this resulted in its isolation in crystalline form.
Thus abscisin II, dormin and lupin flower abscission factor proved to be one and the same compound. At the Sixth International Conference on Plant Growth Substances in Ottawa in 1967, the new name abscisic acid was approved which gives an indication of the compound’s chemical nature.
Q.35. Desrcibe the structure of Abscisic Acid with the help of diagram ?
Ans. Abscisic acid is the trivial name for 3-methyl-5-(1 -hydroxy-4-oxo-2,6,6-trim ethyl-2-cyclohexen- 1 -yl)-cis, trans-2,4-pentadienoic acid. Natural (+)-abscisic acid is optically active. ABA synthesized chemically is racemic and composed of equal amounts of the (+)-and (-)-enantiomers.