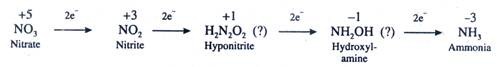ADVERTISEMENTS:
Let us make an in-depth study of the conversion of nitrate into ammonia by the plants.
Nitrogen in nitrate (NO3–) is present in highly oxidised state while in ammonia in reduced form. Therefore, the conversion of nitrate to ammonia is a reductive process. Reduction of nitrate to ammonia takes place in many steps which are mediated by specific enzymes.
At each step two electrons are added and ultimately NO3– in which nitrogen has 5 positive charges is converted into NH3 in which nitrogen has 3 negative charges. The electrons are supplied by reduced coenzyme-I Le., NADH (Nicotinamide Adenine Dinucleotide) and reduced coenzyme-II i.e., NADPH (Nicotinamide Adenine Dinucleotide Phosphate).
(1) Reduction of Nitrate to Nitrite:
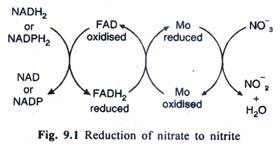
Reduction of nitrate to nitrite takes places in the presence of the enzymes nitrate reductase which requires reduced coenzyme I (NADH) or coenzyme II (NADPH).
This enzyme which is a molybdoflavo protein with an operative sulfhydryl group was first isolated in highly purified form by Evans and Nason in 1953 from soybean leaves and by Nason and Evans (1954) from Neurospora (a fungus). The enzymes isolated from Neurospora could utilise only reduced coenzyme II (NADPH) while the enzyme isolated from soybean leaves could utilise both the reduced coenzyme I (NADH) and II (NADPH).
This enzyme contains FAD (Flavin Adenine Dinucleotide) as its prosthetic group with which is associated molybdenum (Mo). Actual reduction of NO, to NO2– takes place as shown in the Fig. 9.1. Electrons are transferred from reduced coenzyme to FAD which becomes reduced (FADH2). From reduced FADH2 the electrons are finally transferred to NO3– through molybdenum so that N02– and H2O are formed.
(Nitrate reduction takes place chiefly in green leaves and roots. The enzymes nitrate reductase is found in cytosol).
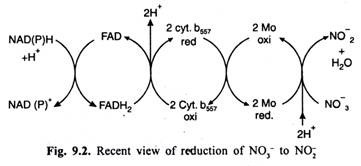
According to more recent findings the enzyme nitrate reductase is in-fact a complex enzyme in higher plants as well as micro-organisms. This complex is an 8 S unit in which NADPH— Cytochrome b557 reductase occurs as a separate (3.7-4.5 S) subunit. The latter is bound to the nitrate reductase by a molybdenum (Mo) binding protein.
The transfer of electrons from reduced coenzymes to nitrate (as shown in fig. 9.2) thus involves two steps:
(i) In the first step, electrons are transferred from NADH/NADPH to cyt. b557 through FAD. This step is catalysed by NADPH—Cytochrome b557 reductase.
(ii) From Cyt.b557 the electrons are transferred to NO3– through Mo in the second step which is catalysed by nitrate reductase of the nitrate reductase complex.
(Cytochrome b and Mo are one electron carriers. Hence, two molecules each of these are required to transfer 2e- through them. Two protons are released when electrons are transferred from reduced FADH2 to Cyt.b while two protons (2H+) are needed in the last place. It is because Cyt.b and Mo can carry only electrons).
The nitrate reductase enzyme is an inducible type of enzyme. Its synthesis is inducted in many plant tissues by NO3–.
In blue-green algae, the source of electrons in the reduction of nitrates is reduced ferredoxin.
ADVERTISEMENTS:
Regulation of nitrate reductase:
As has been mentioned earlier, nitrate reductase is an inducible type of enzyme. It is synthesized de novo in the cells when its substrate i.e., NO3– is present and disintegrates when NO3– is absent. It has been suggested by some workers (Kaiser and Huber, 1994) that nitrate reductase is subject to post-translational modulation involving reversible phosphorylation of some of its serine residues which have regulatory effect on nitrate reductase.
Light, carbohydrate levels and other environmental factors stimulate a protein phosphatase which dephosphoryate some of the serine residues of the nitrate reductase enzymic protein. This leads to the activation of nitrate reductase enzyme.
Under darkness and in presence of Mg++, a protein kinase is stimulated which phosphorylate the same serine residues of the nitrate reductase enzymic protein. This leads to inactivation of nitrate reductase enzyme.
ADVERTISEMENTS:
It is believed that the regulation of nitrate reductase, activity is accomplished in better way and faster (in seconds) through phosphorylation-dephosphorylation process than by synthesis and breakdown of this enzyme which may take hours.
(2) Reduction of Nitrite to Ammonia:
It takes place in the presence of the enzyme nitrite reductase which catalyses the 6e- reduction from NO2– to NH4+ probably with the formation of nitroxyl (NOH) or hyponitrite (H2N2O2) and hydroxylamine (NH2OH) as intermediates as follows:
The immediate source of electrons for the reduction of NO2– is reduced feredoxin in chloroplasts. Reduced pyridine nucleotides act as electron donors in non-green tissues.
ADVERTISEMENTS:
The enzyme nitrite reductase was first isolated by Nason and co-workers from Neurospora and soybean leaves.
Nitrite reductase is found in chloroplasts in leaves. In roots, it is found in plastids.
Nitrite reductase enzyme consists of a single 63 kD polypeptide which contains two prosthetic groups, an iron-sulphur cluster (Fe4 S4) and a specialised heme. These two prosthetic groups are also called as siroheme which probably mediate six-electron transfer as shown in fig. 9.3.
Previously it was thought that the reduction of nitrite to ammonia involved the formation of two intermediate compounds i.e., hyponitrite (H2N2O2) and hydroxylamine (NH2OH) but it is doubtful because:-
ADVERTISEMENTS:
(i) Hyponitrite is quite unstable.
(ii) Hydroxylamine is toxic.
(iii) Moreover, they have never been observed in Free State in the cells.
It is now generally believed that hyponitrite and hydroxylamine may at the best be formed at the surface of the enzyme and leave the surface only when they are completely reduced to further intermediate or ammonia. (NO3 and light induce transcription of m-RNA for nitrite reductase enzyme. Sucrose is known to stimulate this induction while asparagine and glutamine repress it).