ADVERTISEMENTS:
The following points highlight the seven main organic molecules found in the plant cells. The organic molecules are: 1. Nucleic Acids 2. Proteins 3. Organic Acids 4. Carbohydrates 5. Lipids 6. Fatty Acids 7. Secondary Plant Products.
Organic Molecule # 1. Nucleic Acids:
Nucleic acids are polymers of nucleotides. Each nucleotide is made up of a base and a sugar esterified with a molecule of phosphoric acid.
Nucleoside is a base-sugar combination but without phosphoric acid (Fig. 4-3). Nucleotides are either ribonucleotides or deoxyribonucleotides depending upon the presence of a specific sugar, which is ribose in the former and deoxyribose (lacking an oxygen atom) in the latter.
Ribonucleic acid (RNA) is a polymer of ribonucleotides whereas deoxyribonucleic acid (DNA) is a polymer of deoxyribonucleotides.
RNA consists of four organic bases e.g. adenine, uracil, cytosine and guanine whereas DNA has adenine, thymine, cytosine and guanine bases (Fig. 4-2).
Easter bonds link the monomers with the phosphate group from the C-5 of one sugar to the C-3 of the next (Fig. 4-1, 3).
The ribonucleotides are highly important in the major energy transfer reactions during cellular metabolism. In fact, large amount of energy is needed for the synthesis of phosphate esters. When these bonds are hydrolysed large amount of energy is released.
Adenosine series ribonucleotides are the most important ones and some of these include adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP).
It may be added that other ribonucleotides and deoxyribonucleotides also form di-and tri-phosphate esters. The triple esters of the nucleotides combine to form RNA and DNA. Further two phosphate groups are eliminated in the condensation of each nucleotide.
Deoxyribonucleotide combination or deoxyribonucleic acid (DNA) carries most of the genetic information in the cell. It comprises two parallel strands which are tightly linked together through hydrogen bonds between amino and carbonyl groups (C = O) group of adjacent bases (Fig. 4-1). The linking is very specific so that a purine always links with a pyrimidine.
Thus, adenine always bonds to thymine and cytosine to guanine. These pairs of bases are referred to as complementary since exact fit is permitted between complementary bases because of their molecular size and structure. The two strands then coil into a double helix because of hydrogen bonding as depicted in Fig. 4-1.
The double helix is self-duplicating because of similar base pairing and because only complementary bases can pair. Replication of DNA usually occurs by semiconservative process where each half of an original double helix is used as a template. Thus, two exact copies of the original double helix are formed during cell division. Replication may proceed in one or both directions from a given point.
DNA model as proposed by Watson and Crick envisages conservation of genetic information from generation to generation and also synthesis of identical replicate molecules. The general view is that at least three DNA polymerases (I, II, III) are involved in DNA replication; of these DNA polymerase I fill the gaps between Okazaki fragments whereas II and III polymerases remove 3′ → 5′ pieces.
DNA appears to have two main functions: storage and replication of genetic information specific to an organism. It acts as a template for its own replication and also stores coded information which is transcribed into RNA. Tables 4-2, 4-2A show different structural forms of DNA (Fig. 4-3).
The information stored in the DNA is read by the formation of RNA strand which is complementary to that part of the DNA strand forming its template. The RNA formed is called messenger RNA (mRNA); mRNA carries a specific codon which consists of a triplet of nucleotides, and codes for a specific amino acid. There are two additional RNAs present in the cell and these are soluble RNA (sRNA) and transfer RNA (tRNA).
All the three RNAs are essentially involved in accomplishing translation process. Table 4-4 gives a comparison of three RNA types. Table 4-3 Compares DNA and RNA.
Figure 4-4 depicts diagrammatic representation of a set of processes leading from the transcription to final protein synthesis. Each of the tRNA comprises a triplet of bases which is precisely complementary to a codon.
Clearly, each of the specific tRNA has a specific codon which is able to combine with a specific amino acid required by the codon. Through hybridization between single strands of DNA or RNA or DNA-DNA; DNA-RNA; RNA-RNA it is possible to detect the complementary nucleotide sequences.
The required amino acids are lined up in a sequence determined by the mRNA where they are successively linked together chemically and then released from the tRNAs.
Nirenberg and Matthei (1961) artificially synthesized mRNA which comprised poly U (UUU) and was specific for phenylalanine amino acid. Subsequent studies indicated that poly C (CCC) RNA directed formation of poly-proline only. When AAA was used as mRNA only poly-lysine was formed.
ADVERTISEMENTS:
It may be mentioned that UUU was the first triplet code word or codon identified. There are 20 different amino acids as units of translation and triplets of nucleotides only serve as codons for amino acids. Thus nucleotides triplets could form 64(43) different combinations.
Using artificially prepared messengers made up of nucleotides in diverse combinations, as many as 50 codons were recognised. Soon after it became evident that genetic code was degenerative i.e. more than one codon could be concerned with one amino acid.
Further studies revealed that except for tryptophan and methionine, all other amino acids were specified by 2 to 6 different codons. The sequence of nucleotides on the tRNA is called anticodon. For instance, for leucine the codon is CUG and the anticodon is GAC.
The code letter is a nucleotide in DNA or RNA. The sequence of 3 nucleotides in a codon is called code word. Some are nonsense codons and do not code for any amino acid. There are some ambiguous codons which code for more than one amino acids e.g. CCA codes for glycine and glutamic acid. In bacteria the starting amino acid is N-formyl methionine whereas in eucaryotes it is methionine.
ADVERTISEMENTS:
The anticodon for both is UAC while the codon is AUG. AUG is also referred to as the initiating codon and protein synthesis is initiated at this site. On the contrary some of the codons like UAA, UAG and UGA are called termination codons. The genetic code is universal and the sequence of nucleotides on a mRNA molecule is called reading frame.
The multicellular plant develops from a zygote following growth and differentiation. Growth is defined variously either as an increase in dry or fresh weight, or even increase in DNA or proteins.
It may also be considered as non-reversible increase in volume. Growth also involves cell division and cell elongation.
Cell division usually implies mitotic cell comprising several phases (prophase, metaphase, anaphase, telophase, etc.).
ADVERTISEMENTS:
In a division cycle the chromosome is split longitudinally to produce two homologous chromatids.
Thus, from one nucleus, two daughter nuclei are produced. The two daughter nuclei are genetically similar.
In the meristematic tissues, cells undergo cycle of DNA synthesis or undergo mitotic cycle. These are G1 (DNA synthesis or post-mitotic phase), G2 (premitotic phase without DNA synthesis) separated by S phase. The mitotic phase begins after G2 and comprises several subphases like prophase, metaphase, anaphase and telophase.
Chromosomes are made up of DNA which has autocatalytic function e.g., replication.
Similarly enzymes (DNA polymerases) have been isolated which could successfully support DNA synthesis in a cell-free system. These enzymes need DNA building blocks as well as some cofactors.
Active cell division is restricted to meristematic cells. In the fully differentiated cells, cell division is rare or absent. Sometimes process (s) of cell differentiation could be disturbed and thus cell division could continue. In a tissue culture medium, a part of plant could be divided repeatedly. Sometimes tumors are produced due to uncontrolled cell division.
ADVERTISEMENTS:
Much of the work has been done in Nicotiana especially in hybrids involving N. Langsdorffii. The tumors may also be produced by viruses. Agrobacterium is known to induce crown galls. These galls may be produced on any part of the plant following an inflicting of wound.
The entry of bacteria specifically through the wounds is a must for gall formation. Initially a callus is formed after injury and it grows into a tumor. Cell divisions in a gall are not oriented periclinally.
Introduction of 5-FUD in the wounds inhibits gall tumor formation. There is thus synthesis of DNA in a gall and also synthesis of tumor-inducing principle (TIP) released by bacterium. This TIP appears to be bacterial DNA. The ability of cells to divide is under the regulation of the organism. The fact that the cells of plants differentiate in different ways even though they have the same genetic material, is quite intriguing.
In recent years it has become increasingly evident that cells are omnipotent or are bestowed with the genetic totipotency. Isolated single cells from carrot stem or root when grown in tissue culture have been shown to differentiate into callus or even carrot plantlets.
Similarly enzyme-treated cells, where cell wall is removed, produce protoplasts and the latter are grown in full-fledged plants. The protoplasts may be raised from mesophyll cells, fruit wall or even microspores.
All the diploid cells possess genetic information for variable characters. Interestingly not all of these genes are active or functional at the same time. Seemingly specific gene (s) or gene blocks become active at one developmental phase whereas in another tissue yet another set of genes becomes functional. This would explain differentiation at a specific time.
Thus differences in gene activity causing variable differentiation could be attributed to number and position of genes and also their activity. In summary, gene activity may be visualized in terms of transcription or translation. Differential gene activity may affect these two levels and hence variable differentiation may be brought about.
Giant and lambrush chromosomes provide interesting system to demonstrate differential gene activity. Like the salivary glands of diptera insects, giant chromosomes have been reported in endosperm, antipodals, and suspensor cells. These giant chromosomes comprise hundreds of parallel aligned homologous chromosomes.
In fact, each chromatid is divided longitudinally. There is also discernability of transverse bands connected by faintly staining portions of chromatids. Genes are located on these deeply- staining transverse bands. Under the spell of primary activity of genes these bands undergo structural changes.
Consequently their DNA unfolds into a puff like structure or Balbiani ring. The unfolded DNA transcribes mRNA. As will be noticed, these puffs are areas of high transcription or primary gene activity. During the different phases of development, puffs disappear and new ones are formed. In summary, specific “puffing” patterns indicate microscopically visible expression of differential gene activity.
Using biochemical methods it has been possible to demonstrate structural differences in mRNA produced during different phases of growth and differentiation of an organ, seedling, etc. It may be inferred that differential gene activity is manifested at the level of mRNA.
The proteins represent the secondary gene products. Similarly, enzymatic proteins also point towards the same. Using electrophoresis technique with polyacrylamide gels, phase and tissue specific patterns of proteins, different isozymes have been shown. Some of the enzymes where isozyme patterns have been worked out are those of peroxidase, dehydrogenases and amylases, etc.
This is an interesting demonstration of differential gene activity at the proteins levels. For instance, pattern of peroxidase and protein has been shown to vary with the tissue. Obviously differential gene activity controls differentiation. Coupled with their behaviour we need to provide answers on the basis and/or factors causing their activation or inactivation.
In a way we need to understand how gene activity is “switched on” and “switched off’? Else we need to know the type of stimulus which affects the different states of activity of the genes. In general, either internal and external factors or conditions affect regulation of gene activity.
Regulation of gene activity is best explained in terms of transcription (RNA) and translation. As early as 1961, Jacob and Monad proposed a model to explain the regulation of gene activity (Fig. 4-5). As is evident from Fig. 4-5, there are regulator, operator and structural genes. The group of functionally related structural genes, along with operator gene is referred to as an Operon.
In multicellular systems a modification of this model (Fig. 4-6) has been proposed by Britten and Davidson (1969).
The genes could be possibly activated because of the interaction of the 3 types of genes and possibly through substrate induction. Similarly genes could be inactivated through end product repression. It is assumed that repressors are presumably inactivated by low molecular weight metabolites (effectors).
Once the repressor is in activated, the blockage of the operon is released and hence it is activated and thereafter its structural genes code for specific proteins. Obviously, repressor which is produced by the regulator gene, blocks the operator gene and thus the activity of the latter is stopped.
The structural genes transcribe mRNA. The effector has been shown to be a substrate of an enzyme also which is coded by the operon. Sometimes the effector has been shown to be an end product of a reaction or a series of reactions.
In eukaryotes, DNA of chromosomes is surrounded by proteins. Some of the proteins are basic histones. In addition a little amount of acidic and neutral nuclear proteins is also associated with DNA. Histones abound in arginine and lysine. Thus histones may be rich in either of the two amino acids.
Histones have been suggested to participate in the regulation of gene activity. Available evidences have shown that inhibition of transcription is caused by histones and that histones cause specific inhibitory effect. Some workers dispute this suggestive role of histones. Majority of the evidences do support the role of histones in the repression of gene activity or transcription.
In general, histones may be causing contraction of chromosomes and thereby regulating the heterochromatic areas. Such an effect appears to be unspecific. According to second viewpoint specific histones inhibit particular gene locus. Yet another thinking is that histones complex with RNA.
Phytochrome may be playing a vital role in intercellular regulation. A reference was made to the model of Britten and Davidson (1969). The varied developmental processes were shown to be mediated by different phytohormones.
Elsewhere, the recent hypothesis concerning the role of cyclic AMP or even ethylene as second messenger. Accordingly phytohormones would be primary messengers and they possibly induce second messenger.
From amongst the external factors, temperature and light contribute significantly in the regulation of gene (s) activity. Temperature affects RNA production in plant giant chromosomes. Possibly temperature affects metabolic processes.
The role of light of different wavelengths in regulating metabolic and developmental processes. In fact photomorphogensis has received considerable attention in recent years. It seems well established that phytochrome system activates gene activity. In fact, a parallelism has been shown to exist between the external factor light and phytohormone (s) (GA) action.
The possibility of red light in promoting ethylene production has also been suggested. There is a good justification to believe that external factors and phytohormones may be affecting the state of activity of the genes through a second messenger.
Organic Molecule # 2. Proteins:
Proteins are polymers of twenty different amino acids. Structurally and functionally, no two proteins of an organism are similar. The differences exist at the sequence of the amino acids. Differences among proteins cause variations in organs or developmental stages. The structure of protein is best visualized at four levels of organization (primary, secondary, tertiary and quaternary).
Primary structure is the simplest level of organization of protein which is constituted by the polymers of amino acids (Fig. 4-7). Some correlation between the primary structure of nucleic acids and proteins is assumed. The secondary structure is obtained by the folding and intra-molecular hydrogen bonding in the polypeptide while the tertiary structure refers to the three dimensional structure of protein.
The tertiary structure is stabilized by the side chains of amino acids. Sometimes in a protein, several polypeptides may be associated to constitute a complex macromolecule. This is the quaternary structure of the protein and each one of its polypeptide units has its own primary, secondary or tertiary structures. The mechanism of the synthesis of polypeptides i.e. primary structure is well understood.
Figure 4-8 shows basic amino acid structure having an amino group near a carboxylic acid group. To the latter are attached different side groups (R). These side groups are lipophilic or hydrophilic. Proteins are formed from amino acid monomers by the splitting of a molecule of water from the two adjacent amino acids rather than the chemical structure and based on this following types are recognized:
Types of proteins:
One of the classifications takes into account solubility rather than the chemical structure and based on these following types are recognised:
Simple proteins:
These proteins are made up of amino acids only.
These are of six types and described below:
Albumins:
These are water solube and also dissolve in dilute salt solutions. Most of the enzymatic proteins are included in it.
Globulins:
They are insoluble or very slightly soluble in water. They are important enzymatic or storage proteins.
Protamines:
These proteins are rich in arginine and are of low molecular weight. They may be associated with nuclear proteins.
Histones:
They are rich in basic amino acids and soluble in water. They are in the nuclei and associated with nucleoproteins.
Prolamines:
They are rich in proline and insoluble in water. Some enzymes are prolamines.
Glutelins:
They are major storage proteins which are soluble in dilute acid or base. There are also conjugated proteins which besides their polypeptides contain prosthetic group. Their further classification is based on the prosthetic group.
Metalloproteins:
Some enzymes have metal prosthetic group closely associated with the protein moiety e.g., molybdenum in nitrate reductase.
Mucoproteins:
Here it is a combination of protein and a carbohydrate. They may be components of membranes.
Lipoproteins:
Here protein is associated with different types of lipids.
Nucleoproteins:
These are histones associated with nucleic acids.
Chromoproteins:
Here pigments are associated with proteins. For example, chlorophyll-protein complexes, flavoproteins are included in this.
Organic Molecule # 3. Organic Acids:
Plants also contain organic acids which are highly significant in several different kinds of metabolic processes. Some of the important organic acids which participate in the TCA cycle are malic, citric, α-keto-glutaric and succinic acids. In some systems malic and citric acids accumulate in large quantities in the vacuoles of the plants and offer a sour taste to the tissue.
Some organic acids impart flavour to the fruits. Within the cell several metabolic intermediates are also present and some of these serve as substrates for reactions leading to biosynthesis of plant constituents.
For instance, from pyruvic acid, acetyl-CoA is formed which is an important intermediate. This enters the TCA cycle and is metabolized into carbon dioxide and water under aerobic conditions.
Several other plant constituents like gibberellins, carotenoids, steroids, rubber, etc. are formed from acetyl-CoA. The processes of catabolism and anabolism are not haphazard but controlled through several processes which include structure and organization of an organ; competition of metabolites; enzymes, etc.
Organic Molecule # 4. Carbohydrates:
Simple carbohydrates, the first product formed during Calvin cycle are converted into simple carbohydrates through series of reactions.
Carbohydrates not only serve as a source of chemical energy but also furnish the basic carbon skeleton for all other organic substances found in plants and animals.
Originally, carbohydrates were thought to be the hydrates of carbon with empirical formula Cx (H2 O)x or organic substances containing elements, carbon, hydrogen and oxygen, generally in the ratio of 1:2: 1.
Now we know that they are not truly hydrates of carbon because there are several exceptions to this generalised formula, lactic acid has the formula C3 H6 O3 whereas myoinositol has C6 H12 O6 and they are not carbohydrates.
Deoxyribose, a sugar of common biological importance, has the formula of {C3 H10 O4 rather than C3 H10 O5. Moreover, some compounds have general properties of carbohydrates but contain nitrogen and sulphur in addition to C, H and O. Such compounds do not stick to the rigorous definition based on carbon, hydrogen and oxygen composition.
A more appropriate definition of carbohydrates or saccharides is, the polyhydroxyaldehydes or ketones or their derivatives. They can be classified roughly into three main groups: monosaccharides, oligosaccharides and polysaccharides.
Monosaccharides:
These are the least complex carbohydrates or simple sugars which cannot be hydrolysed into smaller units under reasonably mild conditions.
Oligosaccharides:
(Oligo=”few”. These comprise two to ten molecules of simple sugars joined by glycosidic linkage.
Polysaccharides:
These are polymers of monosaccharide units which are joined in a chained fashion or in a branched structure.
If polysaccharides contain recurring units of similar kind of monosaccharides, they are termed as homopolysaccharides; when two or more different monosaccharide units are bound together, then they are called as heteropolysaccharides.
Carbohydrates can also be classified as reducing and non-reducing sugars. The reducing sugars function as reducing agents due to the presence of free aldehyde or ketone groups in a molecule.
They reduce the metal ions like copper and silver in the alkaline solution and ‘Benedicts’ solution is used to detect these reducing sugars.
Optical Isomerism:
This type of isomerism is mostly found in carbohydrates, like the amino acids, they are also optically active and are able to rotate the plane of polarized light due to the presence of asymmetric carbon atom.
We shall consider D-glyceraldehyde, a triose as an initial example of carbohydrate because it has a single asymmetric carbon atom and it can exist in the form of two optical isomer; which are mirror images of each other and are known as enantiomers.
Depending upon the ability to rotate the plane of polarized light to left or right they are classifies as Levorotatory (l or L) and dextrorotatory (d or D), respectively. All naturally occurring sugars are D- form (Fig. 4-16, 18).
Sugars may exist in two forms:
(i) Six membered ring form called pyranose (derivatives of heterocyclic compound pyran) (Fig. 4-17), and
(ii) Five membered form called as furanose (derivatives of furon) (Fig. 4-17).
Some common examples of hexose and pentose with pyranose and furanose forms are given elsewhere.
Monosaccharides:
These are the simplest sugars forming basic building blocks of all the complex carbohydrates. The empirical formula for monosaccharide is (CH2O)n where n=3 or larger. They can be aldehyde derivative (aldose) or ketone derivatives (ketose). Further they can also be classified as trioses (3 carbon), tetroses (4 carbon), pentoses (5 carbon), hexoses (6 carbon), heptoses (7 carbon) and octoses (8 carbons) depending upon the number of carbon atoms (Table 4-5).
Of all these, hexoses and pentoses are most abundant sugars found in plants whereas heptoses are rare. Each of these sugars exists in two series, aldo and keto.
Amongst the pentoses D-xylose and L-arabinose are particularly important constituents of cell walls. D-ribose and 2- deoxyribose are components of ribonucleic acid and deoxyribonucleic acid, respectively. Most common hexose sugar found in an uncombined state is D-glucose (or dextrose). D-glucose is the result of the hydrolysis of starch or cellulose and mostly found in fruits.
D-galactose and D-mannose are the important constituents of cell wall polysaccharides. D-galactose also occurs as a component of oligosaccharide like raffinose, stachyose and lactose.
Another two branched chain monosaccharides, apinose (5 carbon) and hamamelose (6 carbon), have been shown to occur in plants like oleander and bark of witch hazeel. Hamamelose has been found to occur in combination with tannin in Primula species.
All the monosaccharides are soluble in water and sweet in taste but are insoluble in non-polar solvents.
Derivatives of Monosaccharides:
Several different types of derivatives of monosaccharides exist in a plant. We shall be dealing with a few of them.
Sugar Alcohols, Sugar acids and amino sugars:
In sugar alcohols, carboxyl group of the monosaccharide is reduced by H2 gas in the presence of a catalyst or by sodium amalgam in H2O. Sugar alcohols like mannitol, glycerol, sorbitol, etc. are found in nature.
Among sugar acids, three main types are more common aldonic acid, aldaric acid and uronic acid. Aldonic acids are formed when aldehyde group of the aldose sugars are oxidized by some oxidizing agent or by specific enzymes e.g. D-glucose gives rise to D-gluconic acid.
Aldaric acids have both aldehyde-carbon atom and carbon of hydroxyl group oxidized by strong oxidizing agents like nitric acid. Uronic acids are biologically very important. Here, only carbon atom of primary hydroxyl group is oxidized. In plants, D-glucuronic acid and D-galactouronic acid are commonly found as components of cell wall.
Amino sugars which occur as components of glycoproteins have one carbon at 2-position of the hydroxyl group replaced by amino group. Commonest amino sugars are D-glucosamine and D- galactosamine (Fig. 4-19).
Oligosaccharides:
Oligosaccharides are the connecting links between disaccharides and polysaccharides (Table 4-6). Disaccharides are most abundantly found in plant kingdom.
Since the monosaccharides have free and reactive hydroxyl groups, therefore two units or molecules may join by glycosidic linkage to form a disaccharide. The most common disaccharides are sucrose, maltose, gentianose and melibiose. Sucrose is the principal disaccharide present in higher plants and is known as cane sugar.
It is produced either directly as a product of photosynthesis or indirectly from simple sugars in many non-photosynthetic tissues. Carbohydrates are transported mainly in the form of sucrose.
Latter comprises glucose and fructose moieties (O-β-D-fructo furanosyl (2→ 1) α -D-glucopyranoside). Unlike most disaccharides, sucrose has no free anomieric carbon atom and free aldehyde group (Fig. 4-20). That is why it is a non-reducing sugar and does not reduce cupric ions.
It is a disaccharide, product of CO2 fixation during photosynthesis and economically important agricultural commodity, as a nutrient to living organisms. It is a non-reducing, sweet, highly soluble molecule; chemically inert when in contact with proteins since it cannot form covalent adducts with free amino groups.
Sucrose molecules retain a high free energy of hydrolysis-highest known for glycosidic bond. A-glucosyl residue in sucrose is very efficient mode of energy conservation by the plant. Major portion of CO2 fixed photo-synthetically is captured in sucrose. Via sucrose organic carbon is trans-located to different non-photosynthetic organs from green tissues.
It is also used in the synthesis of starch and fructans-storage glycosides. Sucrose which is stored in the seeds is utilized during seed germination and seedling growth. Sucrose is usually stored in the vacuoles and it is estimated that leaf vacuole at mid-day has sucrose nearly as 11 mM whereas cytosol has 55 mM. In the metabolism of sucrose several regulatory factors and enzymes play a vital role.
In the following a simplified scheme of its synthesis is given:
Synthesis of Sucrose:
Sucrose is synthesized in the cytosol and the chloroplast where the Calvin cycle operates. Free glucose and fructose are not the precursors of sucrose.
On the other hand their phosphorylated forms are the precursors. Triose phosphates extruded from the chloroplasts serve as precursor of hexose phosphates and then sucrose.
More details are given below:
The above equation also indicates that only one ATP is required to form the glycosidic bond that connects glucose to fructose in sucrose:
Glucose – 1- phosphate + fructose – 6 – phosphate + 2H2 O + ATP → sucrose + 3Pi + ADP
Since three ATP molecules are needed during the Calvin cycle for each carbon in each of the hexose of sucrose (36 of total ATP), the one additional ATP required to form the glycosidic bond in sucrose is only a minor additional requirement.
F-1, 6-P2 regulates formation of F-6-P and hence sucrose synthesis.
Sucrose phosphate synthase is regulated by several factors e., light, Pi, G-6-P/Pi ratio; F-2, 6-P2. The last factor is a modulatory molecule which regulates sucrose synthesis.
It may be observed that combined action of sucrose-6-phosphate phosphatase and sucrose phosphate synthase (SPS) causes sucrose synthesis.
Recently maize SPS gene promoter has been expressed in transgenic tomato resulting in high sucrose synthesis and more biomass production. It is an excellent example of biotechnology in the application of crop improvement.
Sucrose synthase is a cytoplasmic enzyme and is present in photosynthetic and non-photosynthetic tissues, and helps in sucrose translocation.
Sucrose mobilization:
It may be stated that cleavage of sucrose molecule is pivotal for entering the metabolic pathways of cell.
Following diagram provides a schematic representation of the process:
Invertase enzyme plays a very significant role and the enzyme occurs in cytoplasm, vacuole as well as apoplast. However, it is absent in plastids. The enzyme occurs in multiple forms viz. alkaline (cytosol), acidic (vacuole, apoplast) and neutral.
Transgenic studies have revealed the role of invertase in starch accumulation, organ shape and size and grain filling, in leaves high invertase in vacuole prevents sucrose accumulation in vacuole. Sucrose is shown to control the expression of several enzymes and other proteins. It represses α- amylase, is synergistic and antagonistic for the input of other signals (Pi concentration, auxins, GA, O2 levels).
Other disaccharides like trehalose, maltose, are uncommon (Fig. 4.20). Trehalose is another example of non-reducing sugar mainly observed in fungi, blue green algae and red algae. Now presence of this sugar has also been observed in leaves of fern Botrychium lunaria. It has 2 glucose units joined by α (1 → 1) bond.
On the other hand, maltose, cellobiose sugars are the result of partial degradation of starch (by amylases) and cellulose or lignin (by cellulases) respectively. Two glucose moieties in pyranose form are joined by (1 → 4) in maltose, (1 → 4) in cellulose and (1 → 6) in gentiabiose sugar.
Trehalose protects cells invariably during stress. Its presence in food indicates fungal infection.
It also stabilizes protein against stress denaturation. Lastly disaccharide lactose (O – β – D – Galactopyranosyl- (1 → 4) – β – D Glucopyranose) is found only as milk sugar.
Tri-, Tetra- and Penta- saccharides:
Among trisaccharides raffinose, gentianose and tetrasaccharide stachyose are commonly found in plants (Fig. 4-21).
Raffinose on hydrolysis yields glucose, fructose and galactose and occurs mainly in leaves and storage organs, whereas gentianose on hydrolysis yields two molecules of glucose and one of fructose and found mainly in Gentiana.
A tetrasaccharide, stachyose has been found in several tree species and comprises glucose, fructose and two molecules of galactose (Fig. 4- 21).
This sugar has been found to replace sucrose as transport metabolite in Verbascum thapsus, Fraxinus americana. Large amount of this sugar has been detected in leguminous seeds. Verbacose is only pentasaccharide found in plants.
Polysaccharides:
Simple sugars produced during photosynthesis are not utilized immediately but are converted to high molecular weight compounds, the polysaccharides. Latter are widely distributed in plants (Table 4-7). Being insoluble, polysaccharides can function as reserve substances without disturbing the osmotic equilibrium of the cell and as components of cell wall polysaccharides mainly in leaves, seeds and fruits.
On hydrolysis with specific enzymes or acids, they yield monosaccharides or monosaccharide derivatives.
Depending upon the type of monomeric units, they are divided into two main classes e.g., homopolysaccharides (one type of monomeric unit) and heteropolysaccharides (different types of monomeric units).
Homopolysaccharides are given names according to the type of building blocks e.g. polysaccharides with glucose units (Glucan); with fructose (Fructosan); with mannose units (Mannans). We shall be dealing with storage structural polysaccharides in detail (Table 4-7).
A. Storage Polysaccharides:
I. Glucans (Starch):
Starch is the principal storage carbohydrate in higher plants and is usually stored in the form of granules in the cytoplasm of seeds where it acts as a source of nutrient for the seedling growth. It is also stored in fleshy tuberous roots and woody twigs. It occurs in two forms (i) Amylose and (ii) Amylopectin (Fig. 22).
Amylose is a straight chain polymer consisting of D-glucose units (200-1000 glucose molecule) joined by (1 → 4) linkage. Amylose has both reducing and non-reducing ends and gives blue black colour with iodine. Iodine atom occupies the helical coil of glucose which is formed when amylose is suspended in water.
Degradation of amylose can be completed by amylases (α -and β-) widely distributed in plants, α-amylase hydrolyses (1 → 4) bonds at random giving free glucose and maltose units whereas β-amylase cleaves amylose into maltose units (Fig. 4-23).
The amylopectin is a branched molecule consisting of 2000-22800 glucose units attached with (1 → 4) bonds and side chains are attached with (1 → 6) bonds. It form stable gel like solution (Fig. 4-22, 25).
Molecular weight of amylopectin is 50000 to 1 million in contrast to amylose. Amylopectin can be enzymatically hydrolysed by the combined action of β-amylase and (1 → 6) glucosidase enzymes into maltose and glucose units.
Glycogen, a reserve substance of animals (Fig. 4-22A) is also found in Cyanophyceae, fungi and bacteria and is similar to amylopectin in structure. The difference between amylopectin and glycogen is the profuse branching of glycogen.
Paramylon, a characteristic feature of Euglenophyceae, Ochromonas has been studied recently. It comprises glucose units linked by (1 → 3) β-glycosidic units in a linear chain.
Starch synthesis:
Chloroplasts in leaf and amyloplastids in storage organs have stored starch which has different functions. However, enzymic reactions in their synthesis are similar and a close relationship between starch metabolism and sucrose metabolism exists (Fig. 4-26).
Three enzymes are involved in starch biosynthesis and these are ADP- glucose pyrophosphorylase, starch synthase and branching enzyme. Figure 4-26 gives schematic representation of the process.
ADP-glucose pyrophosphorylase (ADPGPP) present in the plastids with starch and has four distinct subunits; two 55 kDA and 2 60 kDA. Based on the distribution of ADPGPP subunits three groups are recognized: small subunit in all cells; large subunit in non-photosynthetic cells and different large subunits in photosynthetic cells.
ADPG synthesis rate is correlated with the 3PGAP ratio in the plastids and hence starch and photosynthathates partitioning into sucrose in cytoplasm. It may be stated that amyloplastids lack F-1, 6-bisphosphatase and hence cannot produce hexose from triose phosphate. Further changes in Pi levels in amyloplastids influences ADPGPP activity and hence starch synthesis.
A (1 → 4) glucan chain oligosaccharides must be present in sufficient number to act as chain primer and used as acceptors for the growth of amylose through starch synthase reactions. In summary, specific primer molecule is required.
Glucose is transferred from ADP-glucose to the non-reducing end of a pre-existing a (1 → 4) glucan chain through starch synthase. The enzyme exists in two major genetically distinct forms: one bound to the surface of starch granule and second, is soluble fraction of amyloplast. The soluble fraction is concerned with the elongation of amylopectin while the gB enzyme synthesizes amylose using BDP-glucose as glucosyl donor.
Branching enzyme forms branches in amylopectin and closely associated with the soluble starch synthase.
α-(1-4)-glucan → α-(1-6) branched α-(1-4) glucan
As reported in potato, maize, rice and sweet potato the enzyme is usually found in two or three isoforms.
Starch degradation:
Different pathways exist in photosynthetic and non-photosynthetic tissues. In fact dynamic and regulation of enzymes vary.
In the storage organs like tubers starch degrading enzymes are dormant whereas in the germinating seeds the activity level of these enzymes increases enormously.
Hormones like gibberellins play a vital role in the induction of hydrolases. In the photosynthetic tissues
degradation process exists all the time though in a mild state. It is intriguing to mention that all the degradating enzymes are also present in the cytoplasm and their level is higher than plastids. The precise role of these enzymes is not well understood.
Following are the main degrading enzymes:
i. α-amylase — amylose symbol maltose (some glucose, maltotriose) amylopectin symbol short-chain oligosaccharides (Fig. 4-23, 25)
ii. β-amylase — removes maltose residues from non-reducing end of amylose chains
iii. α—glucosidase— removes α-glucosyl groups from terminal position of
iv. α-glucan chain debranching enzyme-capable of hydrolysing α (1-6) branch linkages.
v. α-(1, 4)-Glucanlyase-degrades maltose, malto-oligosaccharides 1, 5- anhydrofrutose
vi. α-glucosyltransferase
The first four enzymes are capable of degrading starch into glucose:
I, 4 glucan phosphorylase participate in the phosphorylytic route for cleavage of starch.
We know that amylopectin component of starch is branched chain molecule. The mechanism by which the side chains are established is not yet known.
An enzyme (Q) has been isolated from potatoes which along with phosphorylase produces α (1 → 6) glucosidic bond. The participation of new enzyme (Q) in the synthesis of amylopectin catalysed by ADP-glucose-starch transglucosidase has not been studied yet.
Oligosaccharide like sucrose can also be converted to starch with the help of nucleoside diphosphate glucose.
The two enzymes sucrose synthase and starch synthase catalyse the conversion of sugar to starch in the presence of ADP.
II. Fructosans or Fructans:
Certain plants do not store starch as reserve material but another type of homopolymer of fructose called fructans, especially in members of Compositae (Taraxicum and Parthenium) and Graminae.
Most commonly studied fructosan is inulin. It is stored in Helianthus tuberosus and Dahalia tubers. Fructosan consists of fructose units linked by (2 → 1) β- glucosidic bond.
All fructans are either inulin type (2 → 1) bond or phlein type (2 → 6) bond. Later are found in Graminae and some microorganism. (Fig. 4-27).
Biosynthesis of Fructans:
Fructans are thought to be synthesized from sucrose. New fructose moieties after separating from sucrose get attached to the fructose units by (2 → 6) β-glycosidic bonds.
Recent studies show that fructose units can also be transferred via uridine diphosphofructon (UDPF).
III. Mannans:
They are mannose homopolysaccharides found in yeast, mold, bacteria and higher plants, comprising chains of mannose units linked by (1 → 4) β(?) bond or (1 → 6) linkages may occur. The structure of mannans has not been studied in details.
B. Structural Polysaccharides:
Structural polysaccharides constitute up to 90 per cent of dry weight of the primary wall. The shape, elasticity and rigidity of the plant cell wall depend upon the polysaccharides they contain.
Although chemical composition of the polysaccharide has been studied in detail, yet data regarding biosynthesis are not sufficient.
I. Cellulose:
Cellulose is the major constituent of cell wall polysaccharides in all the higher plants. It is a linear polymer of glucose units held together by β (1 → 4) linkages (Fig. 4-24).
Recent studies show that native cellulose is made up of approximately 14000 glucose moieties with molecular weight 2-3 millions.
Cellulose being an inert material requires prolonged heating with concentrated H2SO4 for complete degradation into glucose units.
“Saccharification of wood” and its partial hydrolysis into cellobiose can be done by the enzyme cellulase present in bacteria; cellobiose in turn is attacked by the enzyme cellobiase which yields free hexose units.
Cellulose is insoluble in water, lacks chemical reactivity and is incapable of being degraded rapidly, so it lacks nutritive value.
Biosynthesis:
Cellulose synthesis was thought to be accomplished by the enzyme preparation from bacterium Acetobacter xylinum.
Enzyme catalyses the transfer of D-glucose units from UDP-glucose to cellulose. In contrast enzyme preparation from Phaseolus aureus prefers the substrate GDP-glucose.
Transfer is carried out by an intermediate glycolipid (acts as transport) which carries UDP-glucose across lipophilic membranes.
II. Pectic Compounds:
Pectic substances consist of galactouronic acids linked by α (1 → 4) linkage. Three types of pectic substances have been observed in plants.
These are:
(a) Pectic acid:
This has only galactouronic acid in a chain.
(b) Pectins:
These have some methylated carboxyl groups in galactouronic acids.
(c) Protopectin:
This name is used for all the insoluble pectic substances. Not much is known regarding the structure and composition of protopectin.
These pectic compounds play dual role first they are major components of cell wall matrix and second they occur in large quantities in fruits (citrus, apple) from which they are mainly extracted. Being a gelling agent, they are used in food industry. Pectic substances are degraded by pectin esterases (cleave methyl group) and pectinases [break α (1→ 4) glycosidic bond] (See figure 4-27).
Biosynthesis:
The probable starting substance for the synthesis of pectin compound is UDP-galactouronic acid. Latter is formed by the oxidation of the CH2 OH group of sugar. Oxidation of glucose gives glucuronic acid.
Organic Molecule # 5. Lipids:
We have already dealt with two major constituents of higher plants i.e. carbohydrates and proteins. They perform dual function; they act both as structural components of cell wall and as reserve food materials. “Lipids are water insoluble organic biomolecules and can be extracted from the cells or tissues by one of the several non- polar solvents like ether, chloroform and acetone.”
Classification:
Lipids can be classified on the basis of backbone structure into two main categories:
Organic Molecule # 6. Fatty Acids:
Chemically, fatty acids are the union of organic molecules attached to certain alcohol. They are straight chain hydrocarbons containing even number of carbon atoms.
Branched chain fatty acids seem to be absent in higher plants. Among the most predominating saturated fatty acids found in plants are palmitic acid (C16H32O2), stearic acid (C18H36O2) and other saturated fatty acids which are lauric acid (C12), myristic acid (C14).
Among the unsaturated ones, oleic acid (C18), linoleic acid (C18) and arachidonic acid (C20) are present. Linolic and linolenic acids are found in linseed oil, while others are found in cellular organelles (Table 4-8).
In summary the fatty acids are of two types: saturated and unsaturated.
Saturated fatty acids:
These may be considered as based on acetic acid as the first member of the series. Cells synthesize fatty acids from 2-carbon or even-numbered building units of acetic acid. Examples of saturated fatty acids are given in table 4-8. Acetic, propionic, butyric acid, palmitic acid are some important examples of this category.
The carbon atoms of fatty acids are numbered in sequence from the-COOH carbon (C no 1). The second carbon of the acid counted after the carboxyl group is referred to as α-carbon. Carbon no 3 is β-Carbon. Carbon 4 is Ƴ.
Unsaturated fatty acids:
In such fatty acids the melting point is greatly lowered and all the common unsaturated fatty acids of nature are liquids at room temperature. These are further subdivided according to the degree of unsaturation:
Monounsaturated acid:
These FA have one double bond. Examples are oleic acid, linoleic, α- linolenic acid. Oleic acid is most abundant fatty acid in nature.
Polyunsaturated acids:
These FA have two, three or four double bonds.
Two double bonds-linoleic acid, found in peanuts, maize, cotton seeds and soybeans.
Three double bonds-linolenic acid found in linseed oil.
Four double bonds; arachidonic acid found in peanut oil.
Arachidonic acid on metabolism gives rise to prostaglandins and plays a significant role in biochemical activity on smooth muscles, blood vessels and adipose tissue.
Fatty acids are characterized by several important properties and these include melting point; saponification, etc.
I. Triacylglycerols:
Triacylglycerols are also called as neutral fats and are the commonest lipids in plants. “They are the esters of trihydric alcohol, glycerol and long chain fatty acids.”
Triglycerides which are solid at room temperature are called fats whereas those which are liquid at room temperature are called oils.
They are found as plant reserve material in the form of intracellular oil droplets in storage organs like seeds, fruits and sometimes in the chloroplasts also.
II. Phosphoglycerols:
They are also called phospholipids or glycerol phosphatides and are frequently associated with cell membranes. They may also occur in the cytoplasm.
“Phospholipids, as their name indicates, are derivatives of glycerol in which one of the three fatty acids is replaced by phosphoric acid.” The compound having two fatty acids and one phosphoric acid is called phosphatidic acid.
The other wide spread phosphoglycerides in plants are: phosphatidylethanolamine (PEA), phosphatidylserine (PS) and phosphatidylcholine (PC).
The latter is abundantly found in soybean. Phospholipids are also called as amphipathic or polar lipids since they possess polar head in addition to their non-polar tail.
III. Sphingolipids:
They are important membrane components of plants and animals. On hydrolysis they yield one molecule of phosphoric acid, one of fatty acid and one molecule of sphingosine.
In plants and microorganisms phytosphingosine is the major base of sphingolipids. Fatty acids are attached through the amide linkage to the amino group of sphingosine (Fig. 4-29).
Glucocerebrosides (sphingolipids) are found in plant cell as a part of chloroplast membrane. In cerebroside esterified alcohol group is replaced by sugar molecule.
IV. Waxes:
Waxes resemble fats being water insoluble and are solid esters of higher fatty acids with long chain alcohol (24-36 carbon atoms).
Waxes contain higher aliphatic alcohol instead of glycerol. Recently it has been shown that waxes contain other constituents also.
Waxes are found in plants as components of cell wall and on cuticle of leaf and fruit. They can also occur in the cytoplasm e.g., in seeds of Simmondsia californica also.
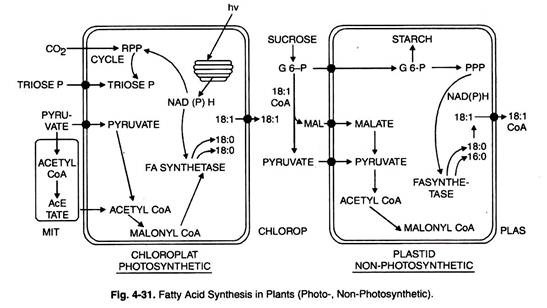
Biosynthesis of Fatty Acids:
The biosynthesis of saturated fatty acids occurs in all the organisms from the ultimate precursor acetyl-CoA.
Complete biosynthesis of fatty acids takes place in cytosol whereas fatty acid oxidation proceeds in the mitochondria.
Overall scheme of reactions is catalyzed by the complex of seven proteins in the cytosol: the fatty acid synthetase complex.
Ultimate source of all carbon atoms of fatty acids is acetyl CoA which is produced from carbohydrates and amino acids.
Acetyl-CoA formed in mitochondria cannot move outside into the cytosol. However, its acetyl group passes through the membrane in some other form.
Citrate produced during tricarboxylic cycle moves to cytoplasm from mitochondria through tricarboxylate transport system.
Acetyl-CoA is regenerated with the help of citrate cleavage enzyme and one molecule of citrate + CoA + ATP is produced. Acetyl-CoA acts as a primer.
Molecules of malonyl-CoA (intermediate precursor of 14 and 16-carbon atoms of palmitic acid) are successively attached to the primer molecule of acetyl CoA accompanied by decarboxylation.
Before starting fatty acid biosynthesis, an important preparatory reaction, the formation of Malonyl CoA takes place in the cytosol in two ways:
There are different systems for synthesis of fatty acids:
A. Extra Mitochondrial system for de novo synthesis (formation of long chain fatty acids):
The cytoplasmic, multienzyme complex converts acetyl-CoA to long chain fatty acids when supplemented with ATP, CO2, Mn2+ and NADPH. The donor of two-carbon units is in the elongation step is malonyl- ACP.
The main reactions in FA synthesis are:
I. Initiating reactions (malonyl CoA formation; acetyl-ACP formation; Malonyl-ACP formation).
II. Principal reactions (condensation; reduction I; dehydration; reduction II).
B. Mitochondrial system for fatty acid synthesis:
Mitochondria have an enzyme system that catalyzes elongation of preformed fatty acids by successive addition of acetyl CoA units.
It may be added that mitochondrial system does not need CO2 fixation and its products are chiefly stearic acid, C20 and C24 fatty acids.
It may be noted that this pathway is essentially the reverse of the pathway for oxidation of fatty acids.
Thus, the system needs the addition of ATP, NADH and NADPH. ADP is required for the formation of acyl-CoA from endogenous fatty acids.
The whole pathway comprised four steps as given below:
In plants vast majority of fatty acid biosynthesis de novo takes place in the plastids (chloroplasts or amyloplasts or leucoplasts).
Compared with plastids the rate of FA synthesis in mitochondria is very low. Briefly it requires two enzymes: acetyl CoA carboxylase and fatty acid synthetase.
In higher plants acetyl CoA in the plastids is of two forms: single large polypeptide and four different polypeptides.
In view of the fact that activity of acetyl CoA is a rate limiting attempts are being made to introduce extra copies of acetyl CoA carboxylase gene to boost fatty acids biosynthesis in oil seeds.
Acetyl CoA carboxylase, an allosteric enzyme catalyzes the rate limiting step in biosynthesis of fatty acids. For its complete enzyme activity it requires citrate or isocitrate as its positive modulation.
Formation of Malonyl CoA and fatty acid synthesis is given below:
Fatty acid Synthesis:
Termination reaction:
Reaction Sequence for Fatty acid Synthesis:
It occurs in three steps:
(a) Initiating reaction,
(b) Chain elongation, and
(c) Termination reaction.
I. Initiating Reaction:
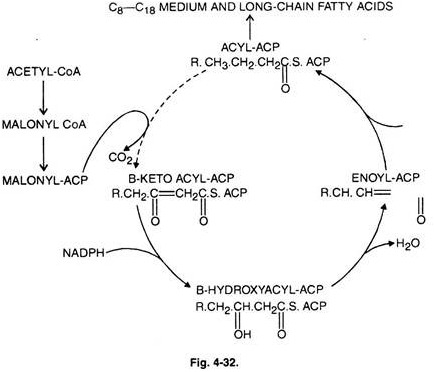
Once malonyl-CoA is formed, further six steps are catalysed by the six enzymes of fatty acid synthetase complex.
The seventh protein has no enzymatic activity itself and is an acyl carrier protein (ACP) to which growing fatty chain is attached covalently.
Initiation reaction starts with the transfer of acetyl group of acetyl CoA to sulphydryl group of ACP by the action of enzyme ACP-acyl transferase.
Then acetyl group from ACP goes to specific cystein residue of the enzyme, β-ketoacyl ACP synthase. Now fatty acid synthetase complex becomes ready to carry the next process of chain elongation.
II. Chain Elongation:
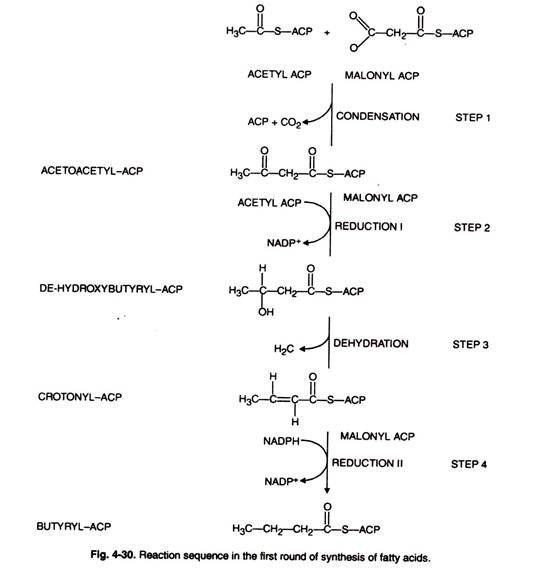
The first reaction (malonyl transfer) is catalysed by the enzyme, ACP- malonyl transferase. Malonyl-CoA formed from acetyl-CoA reacts with sulphydryl group of 4′ – phosphopantetheine arm of ACP to form malonyl-S-CAP with the release of free CoA (Fig. 4-30, 31).
In the next reaction (condensation) acetyl group becomes attached to the malonate residue by the action of enzyme, β-keto acyl ACP synthase releasing free carboxl group. The latter undergoes decarboxylation.
This reaction leads to the formation of 4C-compound; 4-carbon compound undergoes following reductions: 1st reaction, dehydration and 2nd reduction and leads to the production of saturated fatty acids.
Further lengthening of chain step required is acyl transfer. In this fatty acid residue is transferred to—SH group again to which acetyl residues are attached during initiation reaction followed by malonyl transfer and condensation reaction.
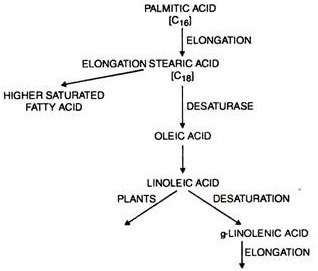
After seven complete cycles palmitoyl-CoA is the end product. Palmitic acid can be converted to stearic acid.
In some of the organisms, the fatty acid synthesis stops with the formation of palmitic acid, production of stearic acid does not occur which has only 2-carbon atom more than palmitic acid.
The specificity of chain elongation depends on the binding site of the enzyme β-ketoacyl synthase which accepts the tetradeconyl group of 14-carbon from ACP but does not accept hexadeconyl group.
Moreover, palmitoyl-CoA may function as feed-back inhibitor of fatty acid synthetase system.
These chain elongation steps are restricted to mitochondria and microsomes since the enzymes involved are bound to this membrane system.
III. Termination Reaction:
When a fatty acid has attained a definite length, terminal reaction product is released from synthetase complex.
Now the latter is able to synthesize new fatty acid molecule. However, the factors which affect chain length and also the time at which the synthesis ceases are not known.
Plant fatty acid synthetase is different from those of animal comprising single protein having six different active sites.
The complete reaction scheme of falty acid synthetase complex is shown in (Fig. 4-32):
The normal products of the plant FA synthetase complex are either C16 FA (palmitic acid) or C18 FA (stearic acid), esterified to an ACP.
In most plant cells FA with chain lengths less than 16 carbons are rare though found in the oil of some seeds like nutmeg.
In such instances the FA synthetase possibly contains an extra medium-chain acyl-ACP thioesterase activity which terminates acyl chain prematurely resulting in the formation of FA with C8, C10, C12 and C14 FA.
Such FA have detergent-like activity. These FA are contained in oil bodies located in the endosperm and embryo of oil seeds.
It is possible to introduce double bonds through desaturates in 18-carbon FA. Thus, stearoyl-ACP desaturase introduces a double bond into the A9 position of stearic acid to form oleoyl-ACP.
The final reactions are the conversions of oleoyl-ACP to oleoyl-CoA and the extrusion of the latter from plastids.
In fact oleoyl-CoA is considered as a central metabolite in oil metabolism in plants. In summary, all the reactions from acetyl-CoA to oleoyl CoA occur in the plastids, the later reactions of oleoyl-CoA take place outside the plastids.
It may elongate further, experience desaturation or have hydroxyl moieties introduced in it to yield acyl- CoAs.
In higher plant cell free systems which synthesize long chain saturated fatty acid has been isolated.
It is still unknown whether fatty acid synthetase present in it is comparable to yeast, bacteria or not, though acyl carrier protein isolated from avogado fruits and spinach leaves are found to be different from yeast.
8 Acetyl CoA + 7 ATP + 14 NADPH → Palmitate
Biosynthesis of Unsaturated Fatty Acids:
Biosynthesis of unsaturated fatty acid has not been completely studied in higher plants. During their synthesis double bonds are introduced into previously formed saturated fatty acids by the enzyme desaturases.
Enzymes acyl-CoA desaturase and stearyl CoA desaturase have been isolated from yeast and Euglena, respectively.
Significant examples of formation of unsaturated fatty acids can be seen in seeds and greening cells.
They are also synthesized during chloroplast differentiation as revealed by the labelling experiments.
They may also undergo secondary conversion of various plant species e.g. conversion of double bonds to triple or additional chain elongation.
Biosynthesis of Natural Fats (Triacylglycerols):
Triacylglycerols which function as storage lipids in higher plants require two major precursors for their synthesis; L- glycerol 3-phosphate and fatty acyl-CoA.
L-glycerol- β-P is derived from dihydroxy-acetone phosphate by the process of hydrogenation in the presence of NADH + H+ or from glycerol by the action of enzyme glycerol kinase as shown below:
Free hydroxyl groups of glycerol 3-P are acylated by two molecules of fatty acyl CoA yielding first lysophosphatidic acid and then phosphatidic acid.
In some microorganism fatty acyl- ACP acts as R donor of fatty acyl instead of fatty acyl CoA. Phosphatidic acid undergoes hydrolysis giving diacyl glycerol with the action enzyme phosphatidate phosphatase.
Diacylglycerol reacts with another molecule of fatty acyl-CoA to form a triglyceride. The reaction is catalysed by the enzyme diacylglycerol acyl transferase. In higher plants, triglyceride may be having 2- 3 different fatty acids.
Reaction sequence is as follows:
Degradation of Fats:
Lipid which acts as a storage material can also be used as an energy rich fuel by the cells to meet their demand for energy e.g. during seed germination, lipid content decreases and their products of degradation are subjected to biological oxidation and then for ATP production.
The degradation of fats starts with the breakdown of triglycerides into glycerol and fatty acids.
The enzymes specifically catalysing fat cleavage are lipases, the hydrolytic enzymes belonging to the group of esterases. The latter cleave ester bonds in the fat molecule along with the uptake of H2O.
Two products, glycerol and fatty acids, undergo various metabolic changes. Glycerol enters the carbohydrate metabolism.
In etiolated cotyledons label from glycerol-1-3-14C can also be degraded to dihydroxyacetone-P and pyruvic acid by glycolytic reactions.
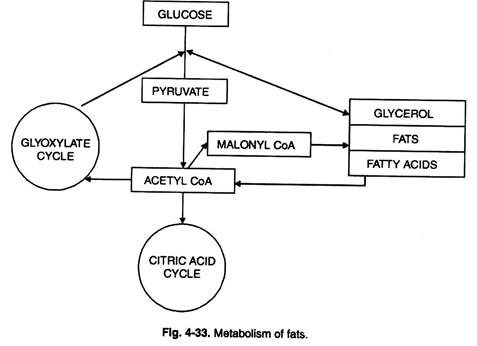
Another cleavage products, fatty acid is subjected to β-oxidation yielding activated C2 fragments : latter serving as substrate for TCA cycle (Figs. 4-33, 34).
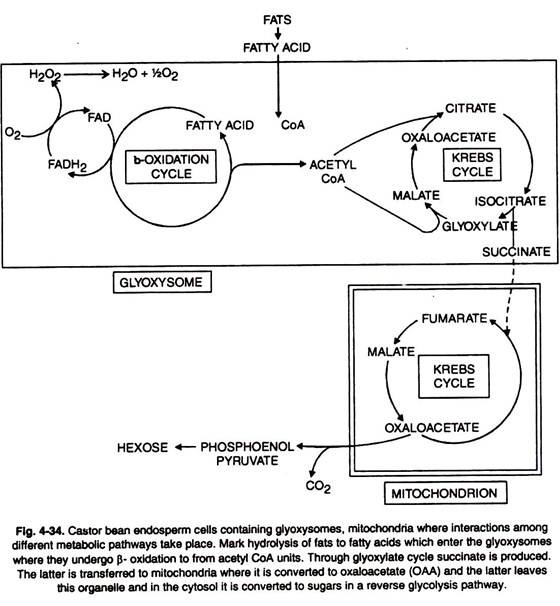
Glyoxylate Cycle:
Several plant structures e.g. pollen and seeds abound in fats. On incubation, these fats are hydrolysed and consumed as source of energy.
This is accomplished through oxidative degradation to be described later on. Fats can also be converted into carbohydrates as follows (Figs. 4-33, 34).
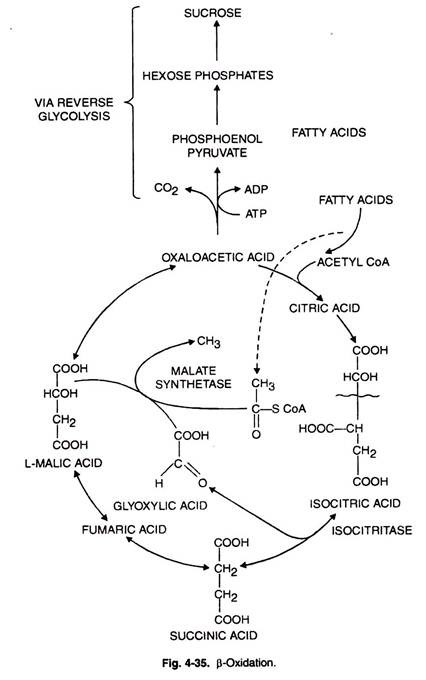
Here isocitrate is broken down to succinate and glyoxylate by isocitrate lyase (ICL). Succinate is converted to malate through TCA cycle.
Fatty acids can also give rise to acetyl CoA. Glyoxylate also gives rise to malate and the key enzyme involved is malate synthetase.
The process increases the malate amount and this in turn increases supply of oxaloacetate. The latter is partially condensed with CoA to give rise to citrate.
The remainder of OAA through series of steps (phosphorylation and decarboxylation) yields PEP which in its turn is converted to hexoses.
The two enzymes malate synthetase (MS) and isocitrate lyase (ICL) are located in glyoxysomes.
The fatty acids produced from fats can be further degraded through β-or α-oxidation depending upon whether β-carbon atom or α-carbon atom are the points of introduction of oxygen function. The two degradative processes are described briefly.
β-Oxidation:
Fatty acids are produced in the cytosol due to their biosynthesis. However, all the enzymes related to β-oxidation system are localized in the inner membrane of mitochondria.
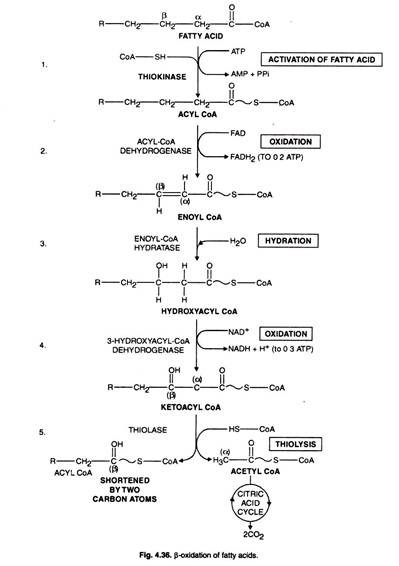
Now the question is how these fatty acids enter mitochondria for their degradation. There are three steps by which the entry of fatty acid is facilitated (Fig. 4-35, 36).
I. Activation of Fatty Acids:
Free fatty acids are esterified with extra-mitochondrial-CoA giving rise to high energy fatty acyl- CoA with the consumption of ATP.
The reaction is catalysed by acyl thiokinase.
R – COOH + ATP + CoA – SH ⇋ RCO – S – CoA + AMP + PPi
II. Transfer to Carnitine:
The acyl group from fatty acyl-CoA is transferred to carnitine, a specific carrier molecule which transports it through the internal barrier of inner mitochondrial membrane to the actual site of degradation.
III. Transfer to Intramitochondrial-CoA:
Fatty acid degradation occurs in matrix and requires acyl-CoA as a substrate and acyl group of fatty acyl carnitine is transferred to intramitochondrial CoA and is converted to acyl-CoA in the matrix.
Following the formation of Acyl-CoA all the reactions of fatty acid oxidation occur in mitochondria in the following steps (Fig. 4-36):
(i) Dehydrogenation Step:
The reaction is catalysed by the enzyme acyl-CoA dehydrogenase. Hydrogen is removed from fatty acid giving α -, β-unsaturated acyl derivatives of CoA.
Four different types of enzymes exist and their specificity depends upon the chain length of fatty acid. They have FAD as prosthetic group. The flavoprotein transfers the electron to the respiratory chain.
(II) Hydration Step:
In this reaction, water is added to unsaturated fatty acyl derivatives of CoA resulting into β- hydroxy fatty acid by the action of enzyme Enoyl CoA hydratase.
The secondary alcohol group of β- hydroxy fatty acid undergoes oxidation in the reaction catalysed by β- hydroxy acyl CoA dehydratase.
(III) Dehydration (2nd) Cleavage Step:
L-3-hydroxy acyl CoA is dehydrogenated to form β- keto acid by the action of enzyme 3- hydroxyacyl-CoA dehydrogenase. β- keto acid being unstable releases C2 fragment as acetyl CoA.
The reaction is catalysed by the enzyme thiolase or is called thiolysis or a thiolytic cleavage. The molecule residue shortened by 2-carbon atom again enters the reaction cycle. The process is repeated until the carbon chain completely splits yielding acetyl CoA. After this another molecule of fatty acid is fed for degradation.
Recently it has been shown that p-oxidation can occur outside the mitochondria e.g. in seeds of Ricinus. The specific enzymes involved are located in the glyoxysomes.
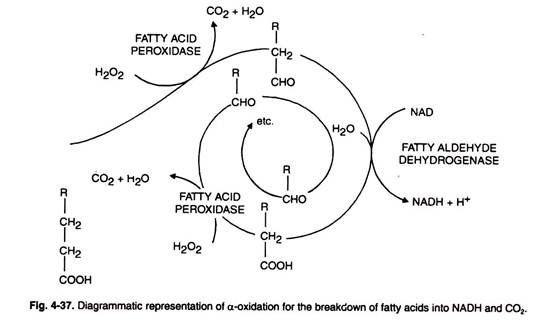
α-Oxidation:
Another mechanism comparatively less important than p-oxidation for the degradation of fatty acid has been observed in cotyledons and young leaves (Fig. 4-37).
In this type of oxidation only free fatty acids serve as substrates. It occurs in the following step:
(i) Carboxyl group of the free fatty acid is eliminated by oxidation with H2O2 by the action of enzyme fatty acid peroxidase.
(ii) The resulting aldehyde compound is further oxidised by a dehydrogenase enzyme.
Degradation of Unsaturated Fatty Acids:
Unsaturated fatty acids are oxidised by the same general pathway as saturated fatty acids except with certain modifications.
The problems of unsaturated fatty acids are that double bonds of unsaturated fatty acids are in cis-configuration whereas ∆2-unsaturated acyl-CoA derivatives have transconfiguration.
Moreover, another problem with unsaturated fatty acid is that removal of 2-carbon atom fragments from carboxyl end yields ∆3-unsaturated fatty acyl CoA rather than Δ2-fatty acyl CoA.
These problems have been overcome by the enzyme enoyl-CoA isomerase which catalyses shift of double bonds from ∆3-cis to ∆3-trans configuration reversibly. The ∆2-trans unsaturated fatty acids act as normal substrate fatty acid oxidation (Fig. 4-38).
Simple Lipids:
Simple lipids contain no fatty acids and occur in the smaller amounts in cells and tissues. Simple lipids are classified in two major classes, the terpenes and the steroids.
Steroids:
They are the derivatives of saturated tetracyclic hydrocarbons, perhydrocyclopentanol pentanophenanthrene containing three fused cyclohexane rings and a single cyclopentane ring.
The precursor for all steroids is triterpenoid, squalene which readily cyclize to form ring structures.
Steroids are widely distributed in the plant and animal kingdoms and those occurring in plants are known as phytosterols (Fig. 4-39). They are present in a very small quantity and their function is not completely understood.
Cholesterol and phytosterol occur rarely in higher plants. They contain other types of sterols like stigma sterols and sitosterol. Another group of sterols present in fungi and yeast are mycosterols. Sterols are not present in bacteria.
Organic Molecule # 7. Secondary Plant Products:
Compared with the primary metabolic products (carbohydrates, lipids, amino acids, proteins, nucleic acids, etc.) there are several secondary metabolic products also characterized in plants and these are phenols, terpenoids, alkaloids, etc. While the former products are essentially required for growth of plants, the latter are accessory plant metabolites and seemingly not involved in the biosynthesis of primary plant metabolites.
They are biosynthesized in a selected few species of plants. Some of the phytohormones (gibberellins, abscisic acid) are terpenoids and belong to secondary metabolites.
Similarly, volatile oils are also a class of secondary plant metabolites. The importance of these secondary metabolites in regulating various physiological processes is well known.
There are many other secondary metabolites e.g., alkaloids, rubber, tannins which are species specific and presumably play some significant role in the biochemical processes within a plant. There still remains vast area regarding their function which deserves attention.
In the followings a brief account of the biosynthesis and functions of some of the important classes of secondary metabolites is given.
Phenols:
The phenols are second largest group of secondary plant products which are characterized by an aromatic ring that is attached to one or more hydroxyl groups.
They have variable constitution viz., simple phenols (hydroquinone), phenol carboxylic acid (gallic acid), phenylpropanes (coumarins, lignin) and flavan derivatives (flavonones, flavones, flavonals). Most of the flavan derivatives occur as sugar esters or glycosides and are found in vacuoles.
In general aromatic systems are synthesized by three different pathways (the shikimic acid, the acetate-malonate and acetate- mevalonate pathways).
Ligin is most widely distributed in plants and perhaps contributed to the transfer of aquatic plant life to land during evolution. Its biosynthesis begins from the cinnamic acids, p- coumaric acids, ferulic acid and sinapic acid through reduction and is converted into corresponding alcohols. The alcohols are transported as glucosides.
Carotenoids and flavan derivatives are some important flower pigments. While the former impart yellow to red colours, the latter compounds give white, yellow, red or even blue coloration. It may be stated that anthocyanins are red and blue flavan pigments.
The pigments may also be divided on cytological basis as follows:
(i) Chymochromic pigments (vacuole or cell sap soluble).
(ii) Plasmochromic pigments (plastidal e.g. chlorophylls, xanthophyll, carotenoids).
(iii) Membranochromic pigments (impregnated in cell walls).
The flower colour is determined by several factors like nature and amount of pigments, pH value, co-pigments, etc.
Phenolics are widely distributed in plants and their precise function is not specifically known. Majority of the phenolics are glycosylated and are, therefore, water soluble. Some of the phenolics are sort of waste products during the amino acids metabolism.
Some of the coumarins inhibit seed germination and even cell elongation by affecting IAA- oxidase. Some of the phenolics behave as phytoalexins and provide defensive mechanisms to plants against pathogens.
Lignin provides tensile strength and cementing of cell walls. The role of flavonoids in insect- attraction to aid pollination is widely acclaimed. Some of the flavonoids stimulate the oxidation of IAA whereas others inhibit such an oxidation.
Terpenoids:
These organic substances are produced from acetyl-CoA via the acetate-mevalonate pathway.
Basically they are constructed from 5C building units.
Depending upon the number of 5C units, the terpenoids are variously sub-grouped e.g.:
In addition tri- and tetraterpenes are also found in plants. Rubber, however, is a polyterpene with n × 5C units.
Hemiterpenes are components of quinones and help in oxidation- reduction reactions. A few monoterpenes are components of essential volatile oils in plants and impart specific odour to plants. These include camphor, carvone and some are antibiotics.
Some of the monoterpenes contribute towards defence or sex attraction signals. Sesquiterpenes include several essential oils as well as ABA.
The role of ABA in dormancy, abscission of leaves and fruits is well known. Gibberellins are diterpenes and contribute significantly towards several physiological processes.
Tetraterpenes include carotenoids and xanthophyll.
Alkaloids:
These are secondary plant products possessing N-containing heterocyclic ring. They are derived from several amino acids like lysine, tryptophan, tyrosine and phenylalanine. To date about 3000 alkaloids have been reported.
Nictotine and nornicotine are found in Nicotiana tabacum.
Phenylalanine and tyrosine are utilized in the synthesis of some alkaloids in several species of Amaryllidaceae and also in Papaver species. Papaver somniferum is a source of papaverine and morphine. Caffeine is found in tea, cocoa and coffee plants.
Most of the alkaloids are sources of drugs. They provide protection to the plant. Conine is obtained from hemlock (Conium maculatum) and was used in poisoning Socrates.
Reserpine is used for curing hypertension and is distributed in Rauwolfia. The latex from opium poppy capsules yields morphine, codeine, and heroin. Quinine (antimalarial drug) and colchicine are some of the other important alkaloids.
Porphyrins:
The biosynthesis of porphyrins is associated with the citric acid cycle and metabolism of amino acids.
Succinyl CoA and glycine are their precursors which combine to produce an intermediate acid called ∆-aminolaevulinic acid as follows:
Succinyl -CoA + glycine δ – aminolevulinic → porphobilinogen → uroporphyrinogen III → Protoporphyrin IX
The above scheme also shows formation of chlorophyll a and chlorophyll b from protoporphyrin IX and also biosynthesis of cell hemins when the latter combines with Fe++.
In blue-green algae open chain tetrapyrroles combine with proteins to form phycobili-proteins. Phytochrome system is a phycobiliprotein. Like the prophyrins, phycobiliproteins are derived from succinyl CoA and glycine.
Some of the amino group carrier present in the cell is pyridoxal phosphate. It is a prosthetic group in starch phosphorylase and transaminase enzymes. In the latter, pyridoxal undergoes reversible transformation into pyridoximine phosphate.
Derivatives of folic acid called tetrahydrofolic acid are also reported. It is also an important coenzyme which comprises peridine, p-amino benzoic and L-glutamic acid. The co-enzyme participates during amino acid and purine metabolism.