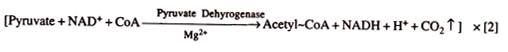ADVERTISEMENTS:
The following points highlight the three steps involved in aerobic respiration in plants. The steps are: 1. Glycolysis 2. Krebs cycle 3. Terminal Oxidation.
Step # 1. Glycolysis (Gk. glycos – sugar, lysis – splitting, Fig. 14.2):
It is also called ЕМР pathway because it was discovered by three German scientists— Gustav Embden, Otto Meyerhof and J. Pamas in 1930.
Glycolysis is the process of partial oxidation of glucose or similar hexose sugar into two molecules of pyruvic acid through a series of ten enzyme mediated reactions releasing some energy (as ATP) and reducing power (as NADH2). It occurs in cytosol or cytoplasm. Glycolysis is common to both aerobic and anaerobic modes of respiration.
ADVERTISEMENTS:
It is the first stage of breakdown of glucose in aerobic respiration and the only step in glucose breakdown in anaerobic respiration. Glycolysis has two phases, preparatory and pay off. In the preparatory phase glucose is broken down to glycerealdehyde 3-phosphate. In the pay off phase the latter is changed into pyruvate producing NADH and ATP.
Preparatory Phase (Energy Spending Phase):
1. Phosphorylation of Glucose:
ADVERTISEMENTS:
Respiratory substrate (glucose or fructose) is formed by hydrolysis of starch or sucrose. Hydrolysis of starch occurs with the help of enzymes amylase and maltase. It yields glucose. Sucrose is hydrolysed by enzyme invertase to form glucose and fructose. Glucose is phosphorylated to glucose-6-phosphate by ATP in the presence of enzyme hexokinase (Meyerhof, 1927) or glucokinase (e.g., liver) and Mg2+.
2. Synthesis of Fructose 6-phosphate:
Glucose-6-phosphate is changed to its isomer fructose-6-phosphate with the help of enzyme phosphohexose isomerase.
Fructose 6-phosphate can also be produced directly by phosphorylation of fructose with the help of enzyme fructokinase.
3. Formation of Fructose 1, 6-Bi-phosphate:
ADVERTISEMENTS:
Fructose 6-phosphate is further phosphorylated by means of ATP in presence of enzyme phosphofructo-kinase and Mg2+. The product is fructose 1: 6 bi-phosphate.
In plants a pyrophosphate (ppi) dependent phosphofructokinase has been discovered which carries out conversion of fructose 6-phosphate into fructose 1, 6- bi-phosphate.
4. Splitting:
ADVERTISEMENTS:
Fructose 1: 6 bi-phosphate Aldolase → splits up enzymatically to form one molecule each of 3-carbon compounds, glyceraldehyde 3- phosphate (= GAP or 3-phosphoglyceral- dehyde =PGAL) and dihydroxy acetone 3-phosphate (DiHAP).
5. Isomerisation of DiHAP:
Dehydroxyacetone 3-phosphate is isomerised to glyceraldehyde 3-phosphate with the help of enzyme triose phosphate isomerase.
Pay Off Phase (Energy Conserving Phase):
6. Oxidation and Phosphorylation:
In the presence of enzyme glyceraldehyde 3- phosphate dehydrogenase, the glyceraldehyde 3-phosphate is oxidised through removal of hydrogen and addition of phosphate from inorganic source to form 1: 3 biphosphoglycerate. NAD+ is hydrogen acceptor. It produces NADH.
7. Substrate Level Phosphorylation (Formation of ATP):
ADVERTISEMENTS:
One of the two phosphates of biphosphoglycerate is linked by high energy bond. It can synthesise ATP and form 3- phosphoglycerate. The enzyme is phosphoglycerate kinase. The direct synthesis of ATP from metabolites is called substrate level phosphorylation.
8. Isomerization:
3-phosphoglycerate is changed to its isomer 2-phosphoglycerate by enzyme phosphoglyceromutase.
9. Dehydration:
ADVERTISEMENTS:
Through the agency of enzyme enolase, 2-phosphoglycerate is converted to phosphoenol pyruvate (PEP). A molecule of water is removed in the process. Mg2+ is required.
10. Formation of Pyruvate:
During formation of phosphoenol pyruvate, the phosphate radical picks up energy. It helps in the production of ATP by substrate level phosphorylation. The enzyme is pyruvate kinase. It produces pyruvate from phosphoenol pyruvate.
Net Products of Glycolysis:
In glycolysis two molecules of ATP are consumed during double phosphorylation of glucose to form fructose 1: 6 biphosphate.
In return four molecules of ATP are produced by substrate level phosphorylation (conversion of 1: 3 biphosphoglycerate to 3-phosphoglycerate and phosphenol pyruvate to pyruvate). Two molecules of NADH2 are formed at the time of oxidation of glyceraldehyde 3-phosphate to 1: 3 biphosphoglycerate.
The net reaction is as follows:
Each NADH is equivalent to 3 ATP, so that the net gain in glycolysis is 8 ATP. Intermediates of glycolysis are used for synthesis of important bio-chemicals.
For example, phosphoenol pyruvate yields shikimic acid which is used in synthesis of amino acids, tryptophan, tyrosine and phenylalanine. Tryptophan is raw material for IAA synthesis. The amino acids are employed for synthesis of proteins, alkaloids, flavonoids and lignin. Similarly, pyruvic acid forms amino acid alanine.
Step # 2. Krebs Cycle or Tricarboxylic Acid Cycle (Fig. 14.3):
The cycle was discovered by Hans Krebs. It occurs inside matrix of mitochondria. The cycle is also named as citric acid cycle (CAC) or tricarboxylic acid (TCA) cycle after the initial product. Krebs cycle is stepwise oxidative and cyclic degradation of activated acetate derived from pyruvate.
Oxidation of Pyruvate to Acetyl-CoA. Pyruvate enters mitochondria through a specific transport protein. It undergoes oxidative decarboxylation to produce CO2 and NADH. The product combines with sulphur containing coenzyme A to form acetyl CoA or activated acetate.
The reaction occurs in the presence of an enzyme complex pyruvate dehydrogenase (made up of a decarboxylase, lipoic acid, TPP, transacetylase and Mg2+). This step is called link reaction or transition reaction or gateway step as it links glycolysis with Krebs cycle.
Acetyl CoA functions as substrate entrant for Krebs cycle. It is also the connecting link between glycolysis and Krebs cycle. The acceptor molecule of Krebs cycle is a 4- carbon compound oxaloacetate. Kerbs cycle involves two decarboxylations and four dehydrogenations (oxidations).
The various components of ten stepped Krebs cycle are as follows:
1. Formation of Citrate:
Acetyl CoA (2-carbon compound) combines with oxaloacetate (4-carbon compound) in the presence of condensing enzyme citrate synthase to form a tricarboxylic 6-carbon compound called citric acid. It is the first product of Krebs cycle. CoA is liberated.
2. Formation of Isocitrate:
Citrate undergoes re-organisation in the presence of iron containing enzyme aconitase first forming cis aconitate and releasing water.
cis-aconitate is then converted into isocitrate with the addition of water while attached to enzyme aconitase. This results in interchange of hydrogen and hydroxyl groups in the molecule.
3. Formation of α-ketoglutarate:
Isocitrate undergoes oxidative decarboxylation in the presence of enzyme isocitrate dehydrogenase and Mn2+. A transient oxalosuccinate is formed as intermediate. It undergoes decarboxylation to form 5-carbon α-ketoglutarate or 2-oxoglutarate. NADH (NADPH according to some workers) is produced.
4. Oxidative Decarboxylation of α-ketoglutarate:
A third oxidative decarboxylation occurs when α- Ketoglutarate is both dehydrogenated (with the help of NAD+) and decarboxylated by an enzyme complex a-ketoglutarate dehydrogenase. The enzyme complex contains TPP and lipoic acid. The product combines with CoA to form succinyl ~ CoA.
5. Conversion of Succinyl CoA to Succinate:
Succinyl ~ CoA is acted upon by enzyme succinate or succinyl CoA synthetase thiokinase to form succinate (a 4C compound). The reaction releases sufficient energy to form ATP (in plants) or GTP (in animals). GTP can form ATP through a coupled reaction.
6. Oxidation of Succinate:
Succinate undergoes dehydrogenation to form fumarate with the help of a membrane based enzyme succinate dehydrogenase. FADH2 (reduced flavin adenine dinucleotide) is produced.
7. Hydration of Fumarate:
A molecule of water gets added to fumarate to form malate. The enzyme is called fumarase.
8. Oxidation of Malate:
Malate is dehydrogenated or oxidised through the agency of malate dehydrogenase to produce oxaloacetate. Hydrogen is accepted by NADP+/NAD+.
Oxaloacetate picks up another molecule of activated acetate to repeat the cycle. A molecule of pyruvic acid that enters a mitochondrion is completely oxidized to form 3 carbon dioxide in one pre-Krebs cycle decarboxylation and two Krebs cycle decarboxylation’s.
A molecule of glucose yields two molecules of NADH2, 2ATP and two pyruvate while undergoing glycolysis. The two molecules of pyruvate are completely degraded in Krebs cycle to form two molecules of ATP, 8NADH2, and 2FADH2.
Significance of Krebs cycle and its Intermediates:
(i) Krebs cycle is the major pathway for the synthesis of reduced coenzymes and controlled release of energy during respiration.
(ii) It is a common pathway of oxidative breakdown of carbohydrates fatty acids, and amino acids (Fig. 14.7).
(iii) Amino acids enter the Krebs cycle directly as glutamate (for a-Ketoglutarate) and asparate (for oxaloacetate) after their deamination,
(iv) Fats produce fatty acids and glycerol. Glycerol is phosphorylated and oxidised to form glyceraldehyde 3-phosphate. Fatty acids undergo p-oxidation to produce acetyl CoA. Acetyl CoA enters Krebs cycle,
(v) Acetyl CoA provides 2-carbon compounds for the synthesis of atty acids, cutin, aromatic compounds and isoprenoids for forming phytol chain of chlorophyll, carotenoids, steroids, terpenes, gibberellins, etc.
(vi) a-Ketoglutarate of Krebs cycle produces an important amino acid called glutamate,
(vii) Succinyl CoA takes part in synthesis of pyrrole compounds of chlorophyll, cytochrome and phytochrome,
(viii) Oxaloacetate produces another important amino acid called aspartate. It also forms pyrimidine’s and alkaloids,
(ix) It forms GTP which is an important component of signal-transduction system,
(x) Krebs cycle is amphibolic (both catabolic and anabolic) because it provides a number of intermediates for anabolic pathways.
Krebs Cycle- An Amphibolitic Pathway:
Amphibolic pathway (Gk. amphi- both, bole- throw) is the one which is used for both breakdown (catabolism) and build-up (anabolism) reactions. Respiratory pathway is mainly a catabolic process which serves to run the living system by providing energy.
The pathway produces a number of intermediates. Many of them are raw materials for building up both primary and secondary metabolites:
(i) Acetyl CoA is helpful not only in using fatty acids in Krebs cycle but is also raw material for synthesis proteins of fatty acids, steroids, terpenes, aromatic compounds and carotenoides.
(ii) a-ketoglutarate is organic acid which forms glutamate (an important amino acid) on amination.
(iii) Oxaloacetate on amination produces aspartate (another important amino acid),
(iv) Both aspartate and glutamate are components of proteins. Pyrimidines and alkaloids are other products, and
(v) Succinyl CoA forms cytochromes and chlorophyll.
Step # 3. Terminal Oxidation:
It is the name of oxidation found in aerobic respiration that occurs towards the end of catabolic process and involves the passage of both electrons and protons of reduced coenzymes to oxygen. It produces water.
Terminal oxidation consists of two processes— electron transport and oxidative phosphorylation….
i. Electron Transport Chain (Fig. 14.4):
Inner mitochondrial membrane contains groups of electron and proton transporting enzymes. In each group the enzymes are arranged in a specific series called electron transport chain (ETC) or mitochontrial respiratory chain or electron transport system (ETS). An electron transport chain or system is a series of coenzymes and cytochromes that take part in the passage of electrons from a chemical to its ultimate acceptor.
The passage of electrons from one enzyme or cytochrome to the next is a downhill journey with a loss of energy at each step. At each step the electron carriers include flavins, iron sulphur complexes, quinones and cytochromes.
Most of them are prosthetic groups of proteins. Quinones are highly mobile electron carriers. Inner mitochondrial membrane possesses five complexes. Complex V is connected with ATP synthesis (F0-F1 particle).
Complexes I to IV are involved in electron transport:
(i) NADH-Q reductase or NADH- dehydrogenase complex,
(ii) Succinate Q-reductase complex,
(iii) QH2-cytochrome с reductase complex, and
(iv) Cytochrome с oxidase complex.
NADH-Q reductase (or NADH-dehydrogenase) has two prosthetic groups, flavin mononucleotide (FMN) and iron sulphur (Fe-S) complexes. Both electrons and protons pass from NADH to FMN. The latter is reduced. However, FMNH2 breaks to release protons (H+) and electrons.
NADH + H+ + FMN → FMNH2 + NAD+
FMNH2→FMN + 2H+ + 2 e– transport chain.
Electron now moves to the FeS complex and from there to a quinone. The common quinone is co-enzyme Q, also called ubiquinone (UQ).
2e–+2Fe3+ S → 2Fe2+S
2Fe2+ S + Q → 2 Fe3+ S + Q2-
Charged ubiquinone picks up protons and passes it into the outer chamber with the help of Cyt b.
FADH2 produced during reduction of succinate also hands over its electrons and protons to ubiquinone or co-enzyme Q through FeS complex. The enzyme is succinate-Q reductase complex.
FADH2 + 2Fe3+ S → 2 Fe2 + S + 2H+ + FAD
2Fe2+ S + Q +2H+ → 2 Fe3+ S + QH2
QH2-cytochrome с reductase complex has three components— cytochrome b, FeS complex and cytochrome с1. Coenzyme Q may also be involved between FeS complex and cytochrome c1. Reduced ubiquinone or ubiquinol (QH2) is oxidised with the passage of protons to the outside and handing over the electrons to cytochrome с via cytochrome b- C1 complex.
Cytochrome c1 hands over its electron to a small protein called cytochrome c. Like coenzyme Q, cytochrome с is also mobile carrier of electrons that transfers electrons between complex III and IV.
Cytochrome с oxidase complex contains cytochrome a and cytochrome a3. Cytochrome a3 also possesses two copper centres. The latter help in transfer of electron to oxygen.
Oxygen is the ultimate acceptor of electrons. It becomes reactive and combines with protons to form metabolic water.
2H++ О2-→ H2O
Energy released during passage of electrons from one carrier to the next is made available to specific trans membrane complexes, which pump protons (H+) from the matrix side of the inner mitochondrial membrane to the outer chamber.
There are three such sites corresponding to three enzymes present in the electron transport chain (NADH-Q reductase, QH2– cytochrome с reductase and cytochrome c-oxidase).
This increases proton concentration in the outer chamber or outer surface of the inner mitochondrial membrane. It creates a proton gradient
The difference in the proton concentration on the outer and inner sides of the inner mitochondrial membrane creates an electric potential across the membrane with inner surface becoming negative as compared to outer surface. The electrochemical potential gradient created across the membrane due to high H+ concentration on one side is called proton motive force (PMF, ∆p).
ii. Oxidative Phosphorylation (Fig. 14.5):
Oxidative phosphorylation is the synthesis of energy rich ATP molecules with the help of energy liberated during oxidation of reduced co-enzymes (NADH FADH2) produced in respiration. The enzyme required for this synthesis is called ATP synthase. It is considered to be fifth complex of electron transport chain.
ATP synthase is located in F1 or head piece of F0-F1 or elementary particles. The particles are present in the inner mitochondrial membrane. ATP-synthase becomes active in ATP formation only where there is a proton gradient having higher concentration of H+ or protons on the F0 side as compared to F, side.
Increased proton concentration is produced in the outer chamber or outer surface of inner mitochondrial membrane by the pushing of protons with the help of energy liberated by passage of electrons from one carrier to another.
Transport of the electrons from NADH over ETC helps in pushing three pairs of protons (5 pairs as per latest estimate) to the outer chamber while two (latest estimate-three) pairs of protons are sent outwardly during electron flow from FADH2 (as the latter donates its electrons further down to the ETC).
Higher proton concentration in the outer chamber causes the protons to pass inwardly into matrix or inner chamber through the inner membrane. The latter possesses special rotating proton channels in the region of F0 (base) of the F0 — F1, particles.
The flow of protons through the F0 channel induces F1, particle to function as АТР-synthase. The energy of the proton gradient is used in attaching a phosphate radicle to ADP by high energy bond. This produces ATP. Oxidation of one molecule of NADH2 produces 3 ATP molecules while a similar oxidation of FADH2 forms 2 ATP molecules.
Balance Sheet of ATP:
There is net gain of 2 ATP molecules during glycolysis and 2 ATP (GTP) molecules during double Krebs cycle. Glycolysis also forms 2 NADH2. Its reducing power is transferred to mitochondria for ATP synthesis.
For this a shuttle system operates at the inner mitochondrion membrane. (i) NADH2 → NAD NADH2. (ii) NADH, FAD FADH2. The former operates in liver, heart and kidney cells. No energy is spent. The second method occurs in muscle and nerve cells.
It lowers the energy level of 2NADH2 by 2ATP molecules total of 10 NADH2 and 2 FADH2 molecules are formed in aerobic respiration. They help in formation of 34 ATP molecules.
The net gain from complete oxidation of a molecule of glucose in muscle and nerve cells is 36 ATP molecules (10 NADH2 = 30 ATP, 2 FADH = ATP, four formed by substrate level phosphorylation in glycolysis and Krebs cycle and two consumed in transport of the NADH2 molecules into mitochondria). In aerobic prokaryotes, heart, liver, and kidneys, 38 ATP molecules are produced per glucose molecule oxidized.
Passage of ATP, molecules from inside of mitochondria to cytoplasm is through facilitated diffusion. Since, one ATP molecule stores 34 kJ or 8.15 kcal/mole, the total energy trapped per gm mole of glucose is 1292 kJ or 309.7 kcal with an efficiency of 45%. The rest of the energy is lost as heat.
ATP (Fig. 14.8)
It is adenosine triphosphate. Adenosine triphosphate is formed of an adenine (a purine), a ribose (a 5-carbon sugar) and a row of three phosphate radicals attached to ribose.
The complex formed from’ adenine and ribose is called adenosine. ATP was discovered by Karl Lohmann in 1929. Its functioning through build up and hydrolysis of high energy phosphate bond was discovered by Fritz Lipmann (1941). Lipmann is called father of ATP cycle.
In ATP the last two phosphate radicals are attached by bonds of high transfer potential. They are also called energy rich bonds. The bond between second and third phosphate radicals possesses an energy equivalent of 8.15 Kcals/mole (7.3 Kcal/mole according to early estimates) while the bond linking the second phosphate radical with the first one has an energy equivalent of 6.5 Kcal/mole.
They are represented by the squiggle sign (~) proposed by Lipmann (1941). The last phosphate can be very easily broken up and synthesised.
The easily available from of energy present in high energy bounds of ATP (and other energy carriers like GTP, UTP or CTP) is know as biologically useful energy. Hence ATP can function as energy currency of the living cells.
Synthesis of ATP:
ATP is synthesised from ADP (adenosine diphosphate) and inorganic phosphate (Pi). The reaction is called phosphorylation. It is endergonic or energy requiring. Phosphorylation is of three types— substrate level phosphorylation, oxidative phosphorylation and photophosphorylation. Substrate level phosphorylation or ATP synthesis is directly linked to the liberation of energy in chemical reactions of respiration.
1: 3-diphosphoglycerate + ADP ⇋ 3-phosphoglycerate + ATP
Phosphoenol pyruvate + ADP → Pyruvate + ATP
Succinyl CoA + ADP + Pi → Succinate + CoA + ATP
Oxidative phosphorylation is linked to terminal oxidation of reduced coenzymes (NADH and FADH2) in respiration. The coenzymes release H+ ions and electrons.
The electrons pass over a series of carriers, called electron transport chain, before combining with oxygen to make it reactive. The energy released during electron transport is used in creating a proton gradient. The proton gradient activates ATP synthase of F0-F1 particles resulting in synthesis of ATP.
Photophosphorylation occurs on the thylakoids of chloroplasts. In the primary photochemical reaction an electron is extruded by chlorophyll a on the receipt of radiation energy. The electron passes over a transport chain of carriers.
Sufficient energy is released when the electron passes between cytochrome b and cytochrome f (cyclic photophosphorylation) or plastoquinone to cytochrome f (noncyclic photophosphorylation). It creates a proton gradient. The latter activates ATP synthase of F0-F1, complex to produce ATP.
Functions:
(i) It can store small packets of energy as soon as the energy becomes available so that wastage of energy is minimised,
(ii) ATP makes energy available at a spot away from the area of release of energy,
ADVERTISEMENTS:
(iii) By its accumulation at a spot, it makes available large and continuous supply of energy for carrying out heavy work,
(iv) ATP releases small amount of energy required for building new chemical bonds during anabolism,
(v) It helps in driving energetically un-favourable processes like absorption of inorganic solutes,
(vi) ATP acts as a phosphorylating agent for activating certain metabolites like sugars,
(vii) It maintains bio-electric potential of cellular membranes,
(viii) ATP energises the membrane carriers for influx and efflux of biochemical,
(ix) It energises the enzyme luciferase in bioluminescent organisms for liberation of light.
Utility of Step-wise Oxidation:
(i) There is a step-wise release of chemical bond energy which is very easily trapped in forming ATP molecules,
(ii) Cellular temperature is not allowed to rise,
(iii) Wastage of energy is reduced,
(iv) There are several intermediates which can be used in production of a number of bio-chemicals,
(v) Through their metabolic intermediates different substances can undergo respiratory catabolism,
(vi) Each step of respiration is controlled by its own enzyme. The activity of different enzymes can be enhanced or inhibited by specific compounds. This helps in controlling the rate of respiration and the amount of energy liberated by it.