ADVERTISEMENTS:
The following points highlight the two main types of photosynthetic pigments. The types are: 1. Chlorophylls 2. Carotenoids.
Type # 1. Chlorophylls:
They are the green photosynthetic pigments. Five types of chlorophylls occur in plants other than bacteria— a, b, c, d and e. Out of these only two chlorophylls occur in the chloroplasts of higher plants, a and b. The amount of chlorophyll b is roughly one fourth of total chlorophyll content. Chlorophyll a is found in all photosynthetic plants except bacteria. Hence, it is termed as universal photosynthetic pigment.
It is also called primary photosynthetic pigment because it performs primary reaction of photosynthesis which involves conversion of light into chemical or electrical energy. Other photosynthetic pigments are thence called accessory pigments.
ADVERTISEMENTS:
They absorb light energy of different wavelengths, broaden the spectrum of light absorption and hand over the energy to chlorophyll a through electron spin resonance. Some of the chlorophyll a molecules function as reaction centres (P700, P680).
Bacteria possess two types of related pigments— bacteriochlorophyll (further of several subtypes) and bacterioviridin (= chlorobium chlorophyll). Bacteriochlorophyll a has an empirical formula of C55H74O6N4Mg and molecular weight of 911.
Chlorophyll a is bluish-green in the pure state. It has an empirical formula of C55H70O5N4Mg and molecular weight of 893. Chlorophyll b is olive green in the pure state with an empirical formula of C55H70O6N4Mg and molecular weight of 907. Both the chlorophylls are soluble in a number of organic solvents but chlorophyll a is more soluble in petroleum ether while chlorophyll b is more soluble in 92% methyl alcohol.
Chlorophyll structure was first studied by Wilstatter, Stoll and Fischer in 1912. It has a tadpole like configuration with a head called porphyrin and a tail made up of long chain alcohol called phytol (Fig. 13.8). Porphyrin head is made up of four pyrrole rings which are linked by methine bridges (—CH=). The skeleton of each pyrrole ring is made up of 5 atoms— 4 carbon and one nitrogen. The latter lies towards the centre.
A nonionic magnesium atom is held in the centre of porphyrin head by nitrogen atoms of pyrrole rings (through two covalent and two coordinate bonds).
The external carbon atoms of the pyrrole rings have been given specific numbers, 1-8. Carbon atoms 1, 3, 5 and 8 have methyl groups (__CH3). Carbon atom 2 possesses a vinyl group (—CH = CH2) while carbon atom 4 has an ethyl group (— CH2 — CH3). Carbon atom 6 is attached to next methine group by a fifth isocyclic ring called cyclopentanone.
Carbon atom 7 is connected to phytol tail through a propionic acid residue. Phytol is an insoluble long chain of carbon and hydrogen atoms with a formula of C20H39OH. It anchors the chlorophyll molecule into the lipid part of thylakoid membrane. Chlorophyll without its Mg-core is colourless and called phaeophytin. It is the early electron acceptor.
Experiment: Light and Chlorophyll are necessary for Photosynthesis:
Apparatus:
De-starched potted plant of variegated Croton, black paper, apparatus for starch testing.
Working:
Take a de-starched potted plant of Croton having variegated leaves (leaves with green and non-green patches). Cover one leaf with black paper. Place the potted plant in sunlight. After two hours, pluck the un-illuminated leaf and one illuminated leaf. Test both the leaves for starch.
ADVERTISEMENTS:
Observation:
The darkened or un-illuminated leaf does not show any blue-black patch. The whole leaf appears yellowish after iodine test. The illuminated leaf possesses both blue-black and yellow patches. The bluish patches correspond to green areas while yellow patches are the ones which were previously non-green.
Inference:
Presence of bluish-black patches in illuminated leaf and their absence in darkened leaf clearly indicates that light is necessary for photosynthesis. In illuminated leaf only green or chlorophyll bearing Parts appear bluish-black showing that chlorophyll is necessary for photosynthesis. The non-green patches do not perform photosynthesis.
ADVERTISEMENTS:
Photoluminescence:
It is the phenomenon of re-radiation of absorbed energy. Photoluminescence is of two types, fluorescence and phosphorescence. Phosphorescence is delayed emission of long wave radiations by irradiated substances which continues for some time after removal of irradiation source.
Chlorophylls show mainly fluorescence. There is some delayed emission or phosphorescence but the same seems to be chemiluminescence or bioluminescence.
Fluorescence:
ADVERTISEMENTS:
It is the property of almost immediate emission of long wave radiations by a substance after having absorbed radiation energy. The substance which can emit back the absorbed radiations is called fluorescent substance. All photosynthetic pigments have the property of fluorescence. Chlorophylls show an outburst of fluorescence (called Kutusky effect) during the first few moments of illumination.
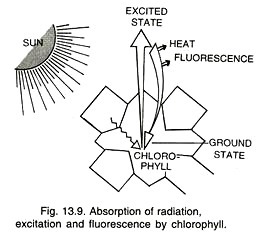
However, most of the fluorescence emitted by photosynthetic organs is due to chlorophyll a because other pigments usually hand over their absorbed energy to it through resonance. On receiving light energy, chlorophyll gets changed to excited state. The excited state lasts for about 10-9 second. If the energy of the excited state is not utilized during this period, it is emitted as heat and long wave radiations.
As some energy is lost during the process of absorption and emission, the emitted radiations are of longer wavelength than the wavelength absorbed by the fluorescent substance. The longer light wavelengths have less quantum energy. Chlorophylls usually show red fluorescence though they absorb blue radiations as well.
Common excited state is called excited singlet state. At times, the electron loses a small amount of energy and stays for some period in the less excited state called triplet excited state.
ADVERTISEMENTS:
Release of energy by the triplet excited state at the time of coming back to ground state is phosphorescence (delayed emission of long wave radiations from an irradiated and activated molecule). Sometimes the electron picks up more energy than the excited singlet state. It is called second singlet state. It remains in this state for a very brief period before coming to excited singlet state.
Emerson Effect:
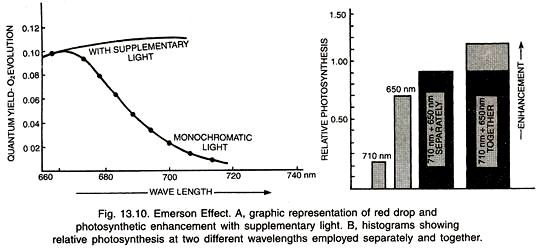
Emerson (1957) excitation and fluorescence by chlorophyll, found a sharp reduction in the rate of photosynthesis when monochromatic beam of more than 680 nm was used alone.
It is called red drop (Fig. 13.10). Emerson (1957) found that rate of photosynthesis can be enhanced if monochromatic beams of two different wavelengths (long and short) were applied simultaneously. It is in excess of sum total of photosynthesis carried out separately by two light beams. The phenomenon is called Emerson effect or photosynthetic enhancement.
It is due to:
ADVERTISEMENTS:
(i) Presence of different types of harvesting molecules around a trap centre in a photosynthetic unit and
(ii) Presence of two interconnected pigment systems with some common pigments.
Absorption Spectrum:
The curve showing the amount of energy of different wavelengths of light absorbed by a substance is called graphic absorption spectrum. It is studied with the help of spectrophotometer.
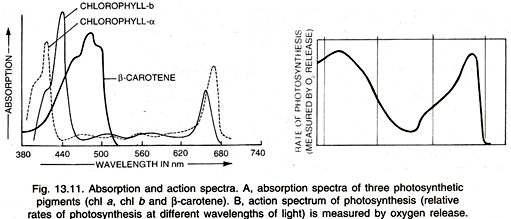
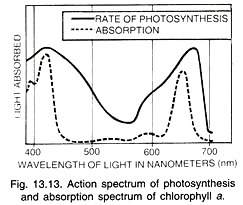
The absorption spectra of chlorophylls a and b (Fig. 13.11) show that they absorb maximum light in the blue-violet and red wavelengths. The pigments are often known after the wavelength which is absorbed to the maximum, e.g., Chl a673 Chl a683 (P680), Chl a703 (P700).
Action Spectrum:
The graphic curve depicting the relative rates of photosynthesis at different wavelengths of light is called action spectrum (Fig. 13.11 B). It shows that maximum photo-synthesis occurs in blue-violet and red parts of the light.
Action spectrum of photosynthesis corresponds closely to absorption spectra of chlorophylls a and b showing that the latter are the main photosynthetic pigments. However, sufficient photosynthesis occurs in the mid part of the light spectrum where carotenoids (carotenes and xanthophyll’s) are active.
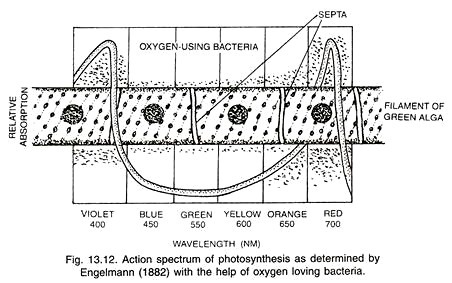
The first action spectrum was studied by Engelmann (1882) by using a green algae which liberated oxygen according to the rate of photosynthesis in different wavelengths of light. Oxygen loving bacteria were used to find out the amount of oxygen liberated (Fig.13.12). It was almost parallel to the absorption spectrum of chlorophyll a.
Type # 2. Carotenoids:
They are a group of yellow, brown to reddish pigments which are associated with the chlorophylls inside the chloroplasts but occur alone inside the chromoplasts. Along with chlorophyll b the carotenoids are also called accessory pigments because they hand over the energy absorbed by them to chlorophyll a. Carotenoids have conjugate double bonds (—C=C—C=C—). They are of two types, carotenes and xanthophyll’s.
Carotenes:
They are hydrocarbons with a general formula of C40H56. Red colour of Tomato and Chillies is due to carotene called lycopene. The most common carotene is β- carotene. It is converted to vitamin A by animals and human beings.
Xanthophylls (= Carotenols):
They are oxygen containing derivatives of carotenes, e.g., C40H56O (cryptoxanthin), C40H56O2 (lutein, zeaxanthin). Yellowish colour of autumnal foliage is due to lutein. The characteristic xanthophyll of brown algae is fucoxanthin (C40H56O6). Both carotenes and xanthophylls are soluble in organic solvents like chloroform, ethyl ether, etc. Carotenes are more soluble in carbon disulphide as compared to xanthophylls.
Functions:
(i) Carotenoids function as accessory pigments. They absorb radiant energy in the mid region of visible spectrum and hand over the same to chlorophyll.
(ii) They protect the chloroplast constituents from nascent oxygen released during photolysis of water. Carotenoids pick up nascent oxygen by means of their double bonds and change the same into harmless molecular state.
(iii) Unquenched excited state of chlorophyll reacts with molecular oxygen to form a highly damaging excited state of oxygen called singlet oxygen (1O*2). Carotenoids prevent this by quenching the excited state of chlorophyll.
(iv) Three xanthophyll’s (violoxanthin, antheroxanthin and zeaxanthin) take part in dissipation of excess energy by conversion of the same into heat.
(v) By their colour, the carotenoids make the flowers and fruits conspicuous to animals for pollination and dispersal.
(vi) β-carotene produces vitamin A in animals.