ADVERTISEMENTS:
In this article we will discuss about the subject-matter and components of electron transport chain.
Subject Matter of Electron Transport Chain:
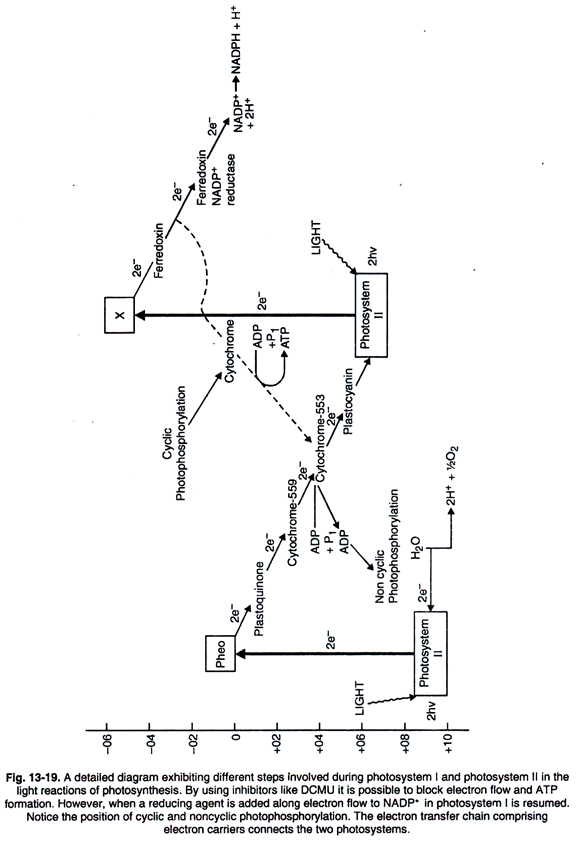
The primary function in photosynthesis is the raising of an electron to a higher energy level in chlorophyll. Then the electron is transferred to an acceptor. It is, as if, there is a hole in the chlorophyll which invites filling. This hole is plugged by electrons from water. Consequently chlorophylls converted to its original ground state.
As will be observed from Fig. 13-19 during PSII reaction, electrons from water are raised from 0.8 to 0.0 volt by energy transfer. Excited chlorophyll receives the electrons from water and then transfers to reaction centre chlorophyll.
ADVERTISEMENTS:
Then these electrons are passed on to some electron acceptor. Pheophytin (Phe) is one of the such acceptor compound. The electron transport components of photosystem I are shown in Fig. 13-20, 22.
The two photosystems appear to function in a connected sequence. This is referred to as the Z scheme and 1960s elaborated this.
ADVERTISEMENTS:
Following evidences could be mentioned for the existence of Z scheme:
1. The occurrence of two separate light reactions.
2. Both cytochrome bƒ–ƒ and plastoquinone (PQ) are oxidized by far red light and reduced by light of shorter wavelength. In PSII, DCMU inhibits oxygen evolution and also inhibits the reduction of cytochrome ƒ by light of shorter wavelength.
3. Even in the presence of DCMU the photoreduction of NADP+can be replaced through an artificial electron donor.
Components of the Electron Transport Chain:
Manganese Protein (Y):
It is believed that PSII contains a manganese protein (Y) which possiblycatalyses the early stages of O2 evolution. Four hundred molecules of chl contain 5-8 atoms of manganese, and about 4 atoms of Mn are required for full O2 evolution.
Chloride Ions:
Chloride ions are possibly associated with the oxidizing side of PSII. Chloroplasts depleted of Cl– ions lose the ability to utilize H2O as an electron donor.
ADVERTISEMENTS:
Z:
Plastocyanin (a copper protein; E0 = 370 mv).
About one molecule of it is present per 400 chl molecules.
P690:
ADVERTISEMENTS:
It is also designated as P690, P680 or P682. It is the reaction centre of photosystem II. It is considered as a molecule of Chla in PSII which traps photons harvested by antenna of chlorophyll molecules. It also exists as a complex with Z and Q.
Pheo:
Once the electrons are transferred to Pheo, the electrons at 0.0 volt are passed along a carrier chain in a downhill fashion (20-1982). Thus energy is lost in this down movement sequence. There is also formation of ATP from ADP in a coupled reaction.
Then the electrons from cytochrome 559 pass to plastoquinone or vice versa, then they are passed on to cytochrome ƒ and then to plastocyanin. These electrons are accepted by P 700 at + 0.4 volt. In the PSI pathway P 700 throws away electrons which are accepted by a high potential substance and then pass on to ferredoxin reducing substance.
ADVERTISEMENTS:
Then the electrons are transferred to ferredoxin and on to ferredoxin NADP reductase enzyme. The ultimate step involves reduction of NADP+ to NADPH conserving chemical energy. As will be observed from Fig. 13-22, 23 it is evident that during transfer of electrons and protons from H2O, initial oxidants and reductants are passed through several steps giving rise to the formation of NADPH and ATP.
Both NADPH and ATP are used in the reduction of carbon dioxide to carbohydrates in the subsequent thermochemical reactions of calvin phase of photosynthesis. Most probably energy for ATP synthesis is given out between cyt. b559 or plastoquinone and cyt. ƒ.
Quinones:
A group of quinones exist in chloroplasts and these are naphthoquinones (vitamin K) and tocopherolquinones (vitamin E). Some plastoquinones (plastoquinone (PQ E0 =+ 0.113V) and plastoquinones A (PQA). It accepts electrons from Q. 3 (3, 4-dichlorophenyl)—1, di-methylurea (DCMU) blocks, electron transport system between Q and PQA.
Cytochrome f:
It is a c-type cytochrome having absorption maxima in the range of 550-555 nm. Nearly one molecule per 400 Chi molecules is present. It carries a single electron and is associated with PSI.
Iron-Sulphur protein (E’ = 290 mV):
This is located between plastoquinone and cytochrome f.
Pigment 700:
It is the reaction centre of PSI and is the modified form of Chla which exists as a dimer. About one molecule per 400 Chi molecules are present. It carries a single electron.
ADVERTISEMENTS:
X (P430):
It is the primary electron acceptor in PSI. The precise chemical nature of this compound is not known but it has a potential more negative than ferredoxin and possibly as low as 600-700 mV. Two Fe-S compounds designated as B and A are considered as intermediate between X and ferredoxin.
Ferredoxin (FD) (E’ = – 430 mV):
It is a protein which contains iron and sulphur and nonheme. Here iron is not associated with heme and is of low molecular weight. Feredoxin of higher plants contains only two iron atoms. The iron is reduced and oxidized by accepting and donating the electron respectively. It carries single electrons.
Flavoprotein Ferredoxin-NADP+ Oxido- Reductase (E’ = -380 mV):
ADVERTISEMENTS:
This enzyme reduces NADP+ to NADPH. It also acts as a transhydrogenese (NADPH2 NAD+→NADP+ + NADPH2) and a diaphorase. It contains one molecule of FAD.
Cytochrome b6:
In these proteins iron is associated with heme. Its redox potential is near zero. Its spectroscopic properties are also similar to cytochrome b of mitochondria.
Nicotinamide Adenine Dinucieotide phosphate (NADP):
It is the terminal acceptor of photosynthetic electron transport in the Z-scheme. In the RPP pathway, NADPH2 donates electrons to 1, 3—diphosphoglycerate. NADP+ does not pass through the chloroplast envelope.
Formerly NADP was regarded as the primary acceptor. Recent studies have shown ferredoxin with a redox potential of nearly 0.42 volt as the acceptor. Later studies have indicated that compounds X and Z precede ferredoxin and are strong reductans.
Three iron-containing proteins called cytochromes are found in chloroplasts. They contain iron as Fe++ or Fe+++ as part of heme prosthetic group. These are cytochromes wt b6 and wt b3;
Cytochrome: (E0‘ = + 4.055 V). It may lie outside the chain.
Cytochrome b 559 (E’ ― 370 mV) It is a high potential form of wt b6.
 Quantum Requirement of the Z-scheme:
Quantum Requirement of the Z-scheme:
Z-scheme requires an input of one photon for each electron moved through each photosytem.
Thus 8 photons shall be required per O2 evolved.
Process of Photosynthetic Phosphorylation:
Two separate and alternate pathways of electron transfer away from the chlorophyll molecule exist. These are cyclic and non- cyclic photophosphorylation (Table 13-3). In the former, the electron released by the chlorophyll molecule does not return to it.
In this process water splits into hydrogen ions, oxygen, and both ATP and NADPH2 are produced. The latter pathway comprises the return of the electron finally to the chlorophyll molecule itself which acts as electron acceptor. In this process, only ATP is produced no splitting of water and no evolution of O2 occurs (Fig. 13-24). Table 13-3 shows comparison of two photo-phosphorylating systems.