ADVERTISEMENTS:
In this article we will discuss about the anatomy of anther.
1. Anther Initiation:
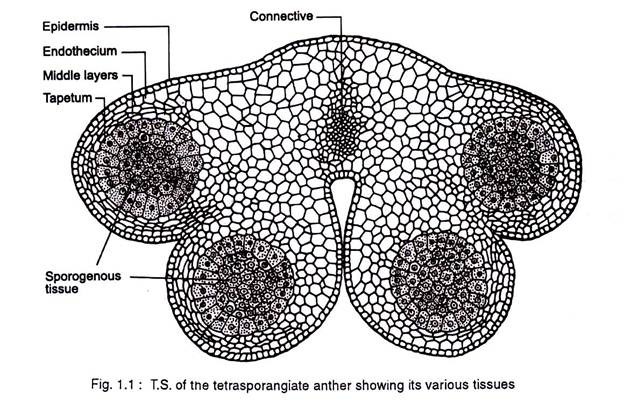
The dynamics of anther development and differentiation depend on the co-operative interactions between cell populations. The anther normally consists of two lobes, each with two elongated microsporangia or pollen sacs. The anther lobes are fused together by the connective tissue (Fig 1.1).
The filament connects the anther to the receptacle in the flower. A differentiated anther has several highly specialized cells and tissues that are responsible for carrying out non reproductive functions and reproductive functions.
ADVERTISEMENTS:
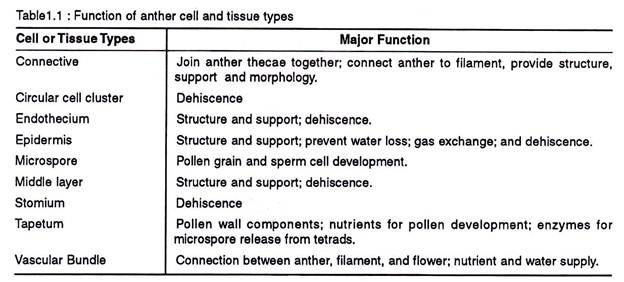
The non-reproductive tissues include the epidermis, endothecium, tapetum, circular cell cluster, connective, stomium, and vascular bundle. Each of these tissues and cell types carry out specialized task (Table 1.1.) In addition to these diploid sporophytic tissues, the anther also contains haploid microspores that fill the pollen sacs and differentiate into pollen grains.
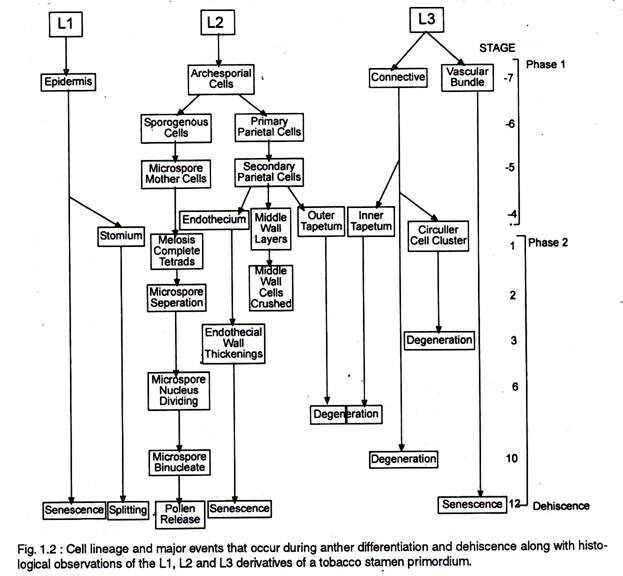
The anther first appears as a cylindrical structure composed of a mass of meristematic cells, which consists of three “germ’ layers designated as L1, L2 and L3, which gives rise to different anther tissues. Thus ones specified the development fate of L1, L2, and L3 layer derivatives is fixed (Fig.1.2.).
In most cases individual tissues and cell types are derived from a single germ layer. For instance L1 layer gives rise to the epidermis and stomium. The former is greatly stretched and flattened in a mature anther. The stomium is located between the two locules of each anther lobe, and the cells in this region are thin walled and in the form of a longitudinal slit.
The L2 layer gives rise to the archesporial cells, microspore mother cells, endothecium, and middle wall layers that lie between the epidermis and the tapetum. The archesporial cells are hypodermal in origin and consist of one to more vertical rows of large cells with dense cytoplasm and deeply staining nuclei. The cells divide periclinally into primary parietal cell toward the periphery and sporogenous cell toward the inside (Fig. 1.3).
The parietal cells undergo a series of periclinal and anticlinal divisions to form two to five concentric layers of anther wall [endothecium, middle wall layers (three layered) and outer tapetum]. The L3 layer gives rise to the connective, vascular bundle, and circular cell cluster adjacent to the stomium.
Both the L2 and L3 layers contribute to tapetum formation. Tapetal cells along the upper portion (inner) of the pollen sacs are specified from the L3-derived connective tissue, whereas those that line the lower portion (outer) of the pollen sacs are specified from the L2- derived archesporial lineage.
Archesporial cells destined to differentiate into microsporangia and surrounding tapetum and endothecium tissue, arise simultaneously in each corner of the anther primordium, while the vascular tissues differentiate within the centre of the anther primordium and establish a connection with the filament.
Thus specific regions or territories are established early in another development within which unique histo-differentiation events occur in a precise chronological order.
The territory based differentiation of four microsporangia with identical tissue patterns and the differentiation of the tapetal cells within each territory from two different cell lineages (Fig 1.2) suggests that cell to cell communication process may play an important role in the anther histospecification process.
For example, it is possible that a gradient of morphogens (these are secreting signaling molecules that play a key role in the formation of the shape and size of organs), synthesized by archesporial cells and interpreted by contiguous L2 and L3, triggers the differentiation of tapetal and archesporial cells.
ADVERTISEMENTS:
Other morphogens appearing later at the boundaries of the microsporangia could be perceived by neighbouring LI-derived epidermal cells and L3 derived connective cells and cause them to differentiate into the stomium and circular cell cluster, respectively.
2. Anther Wall:
Epidermis, endothecium, middle wall layers (2-3 layered), and the tapetum (outer and inner layers) constitute the anther wall.
i. Epidermis:
The epidermis is a single layered protective sheath of the anther. It divides anticlinally and tries to keep space with the enlarging internal tissues of the anther. As a consequence they undergo considerable stretching in surface area. It provides the structural integrity to the anther, assists in gaseous diffusion, prevents moisture loss, and in the dehiscence of the anther lobes.
ii. Endothecium:
The outer most layers of the descendants of the parietal cell located immediately below the epidermis are called the endothecium. It attains the maximum development before the dehiscence of the anther.
ADVERTISEMENTS:
The cells are radially elongated and decorated with fibrous bands (absent in certain members of Hydrocharitaceae and some cleistogamous flowers) that run upward from the inner tangential walls, ending near the outer wall of each cell as an incomplete ring.
The outer tangential walls remain thin. The endothecium is associated with high proportion of α-cellulose and small amount of lignin at maturity. The specialized nature of the endothecium together with the stomium helps in the dehiscence of the anther.
iii. Middle Layer:
The cells of the middle layer are usually ephemeral and become flattened and crushed by early meiosis in the pollen mother cell. However, the layers persist in Ranunculus and Lilium, and the layer adjacent to the endothecium may even develop fibrous thickenings. In few instances it also serves to store starch that is later mobilized to the developing pollen. (Table 1.1)
iv. Tapetum:
The tapetum is the innermost layer of the anther wall and is usually derived from the parietal layer. However, it may have a dimorphic origin in a few species, viz., in Alectra thomsoni the inner tapetum consists of larger cells that is derived from the cells of the connective, whereas, the outer tapetum of smaller cells is derived from the parietal layer.
ADVERTISEMENTS:
Further in Antirrhinum majus and Impatiens glandulifera the tapetum has its origin from the peripheral cells of the sporogenous tissue. Maheshwari (1950) and Echlin (1971) believe in the parietal origin of the tapetum as obligatory.
The tapetum surrounds the sporogenous tissue and attains maximum development when the microspores are in the tetrad stage, after which they go into decline that results in the collapse of the cells. Generally it is single layered but it may divide and become biseriate as in Pyrostegia, Tecoma and Magnolia. While a multiseriate condition is known in Combretum grandiflorum and Oxystelma esculentum.
A. Characteristic of Tapetal Cells:
The cells of the tapetum are characterized as:
ADVERTISEMENTS:
a. They are distinctly enlarged and always ephemeral.
b. The cytoplasm is rich in ribosomes, mitochondria, E.R., many vesicles and active organelles.
c. Cells may be multinucleate or polyploid and are comparatively rich in DNA.
d. There is irregular mitotic divisions and nuclear fusion.
e. They are characterized by rapid and intense activity with degeneration of their cytoplasm.
However the most distinctive feature of the tapetal cells is the ER-Golgi complex, which makes an essential portion of their ultra-structural repertoire, along with secretary vesicles that lie toward the side facing the anther locule and small vacuoles containing lipophilic substances.
ADVERTISEMENTS:
B. Behaviour of the Nucleus in the Tapetal Cells:
Tapetal cells undergo dynamic fluxes during their short life span. The characteristic cytological feature of the tapetal cells, irrespective of the type, is the increase in the content of their DNA, which is initiated with meiosis in microsporocytes and extends through the meiotic division.
Since DNA increase is not followed by regular mitotic division it results in certain cytological abnormalities, like multinucleate cells, endomitosis, polyploid nuclei, polyteny and endoreduplication.
The different stages of nuclear behaviour are:
1. Endomitosis:
It is a condition where chromosome duplication and chromatid separation take place within the intact nuclear membrane and without the formation of a spindle. The consequence is the formation of a large polyploid nucleus. In Cucurbita pepo the uninucleate tapetum has four nuclear sizes which correspond to their degree of ploidy, i.e., 2n, 4n, 8n, and 16n, which is due to endo-mitosis.
ADVERTISEMENTS:
2. Multinucleate condition:
It is a common feature of the tapetal cells, where the nuclear division is synchronous in amoeboid type and asynchronous in secretory type of tapetum and is not accompanied by cytokinesis. Based on the number of nuclear divisions, cells may have 2, 4, 8, or 16 nuclei. In case of nuclear fusion cells outside the expected series of nuclei number may appear.
3. Restitution nuclei:
Mitosis is normal up to the early stage of anaphase, from then onward the two chromosome sets are included within a common nuclear membrane, thus forming a restitution nucleus.
4. Polyteny:
It is a case of increase in chromonemata number per chromosome, thus there is alternation in chromosome number per nucleus.
C. Tapetal Membrane:
A thin and tapetally secreted sporopollenin membrane lines the locule during the final stages of anther maturation. This acetolysis- resistance membrane when deposited on the inner tangential face of the tapetum is called the tapetal membrane or the orbicular wall.
However, in few members of Asteraceae and Gymnosperms this membrane develops on the outer tangential face of the tapetum and is referred as peritapetal membrane. In addition to sporopollenin, it contains insoluble polysaccharides, namely callose, and pectin.
At the time of tetrad formation the tapetal cells begin to form specific spherical bodies called pro-orbicules (Fig 1.5) At this stage they appear between the plasmalemma and the disappearing wall of the tapetum. Later at the same time the sporopollenin is deposited on the sexine baculae and also coats the pro-orbicules, which form the orbicules.
In Sorghum bicolor, the orbicules and a reticulum produced by sporopollenin form an orbicular wall which coats the inner tangential surface of the tapetum. Ultrastructural studies of the membrane in grasses have shown that it has an outer fenestrated layer followed by an irregular network of beaded strands forming complex webbing around the orbicules. The former layer persists through anther dehiscence, while the latter become disorganized before the anthesis.
In Pinus banksiana the peritapetal membrane is formed when the microspores are undergoing meiosisll. It involves the deposition and polymerization of sporopollenin precursors upon a lipoidal primary layer formed by the discharge of unit membrane bound vesicles derived from the dictyosomes.
The function of peritapetal membrane is not clearly understood, however, it is presumed to be related to the following functions:
1. Since it is predominantly made of sporopollenin, it may resist the free passage of materials into and out of the spore mass.
2. The peritapetal membrane is considered to form a kind of impermeable “culture sac”, enclosing the young spores and the tapetal Plasmodium during the period of sexine growth.
3. In members of the subfamily Cynanchoideae of Asclepiadaceae, the tapetal membrane holds the pollen together in aggregate and assist in their collective dispersal.