ADVERTISEMENTS:
In this article we will discuss about the structure of various energy-rich compounds with the help of diagrams.
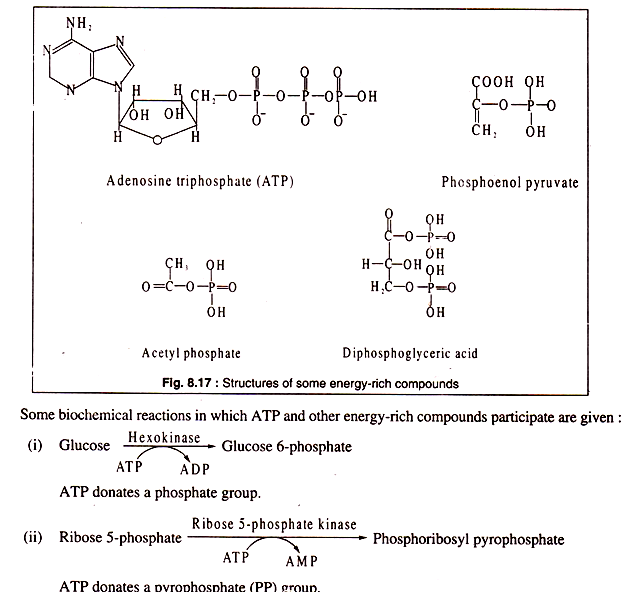
The chief energy-rich compound of all biological cells is adenosine triphosphate. ATP consists of adenine, ribose and three phosphoric acid molecules. ATP is energy-rich because its terminal phosphate group can be hydrolysed to release energy.
Many biochemical reactions are endergonic in nature and cannot occur spontaneously unless energy is supplied. When such a reaction is coupled with another reaction which is exergonic in nature, then only can the reactants be enzymatically driven to give the products.
ADVERTISEMENTS:
Hydrolysis of ATP is the most common exergonic reaction coupled to drive an endergonic biochemical reaction. When ATP is hydrolysed, the quantity of energy set free (∆G) varies depending on pH, concentrations of Mg++ and ADP etc. Under standard conditions (∆G°) the free-energy of ATP hydrolysis ATP –> ADP + Pi is taken to be -7.3 K cal/mole of ATP.
Besides ATP, other energy-rich compounds in biological cells are also present, such as guanosine triphosphate, uridine triphosphate, cytidine triphosphate, phosphoenol pyruvate, acetyl phosphate etc.
Free energy released by these compounds is shown in Table 8.1:
ADVERTISEMENTS:
The structures of ATP and some other energy-rich compounds are shown in Fig. 8.17:
From the above examples of biochemical reactions, several points may be noted. Firstly, in some reactions the energy liberated by hydrolysis of ATP can be used for transfer of one phosphate group, two phosphate groups, or the adenylic acid (AMP) to acceptor molecules to make them energetically more reactive.
Secondly, the energy rich phosphate group of some compounds may be utilized for synthesis of ATP itself, presumably the energy liberated by these compounds is more than what is needed for joining the terminal phosphate group of ATP.
Though there are several energy-rich compounds in biological cells, ATP is the most commonly used source of energy for biochemical reactions. ATP is a comparatively large molecule and it cannot be stored in large amounts in cells, particularly in small cells of bacteria.
Hence, it is constantly synthesized and consumed. The cells generally store energy in smaller molecule, like glucose, which are broken down and the energy released by breakdown of the chemical bonds is utilized for generation of ATP.
ATP may be formed in cells in three different ways by adding a phosphate group to ADP accompanied by withdrawal of one molecule of water. The process is called phosphorylation. The three methods of ATP generation are substrate-level phosphorylation, oxidative phosphorylation and photo-phosphorylation.
ADVERTISEMENTS:
In substrate level phosphorylation, an energy-rich compound — such as phosphoenol pyruvate or di-phosphoglyceric acid or acetyl phosphate — donates its phosphate group to ADP to form ATP. This type of phosphorylation is the only way to generate ATP in organisms carrying out a fermentative metabolism. In oxidative phosphorylation, ATP is generated in electron transport system (ETS) when protons (H+) move across the membrane producing a trans-membrane proton gradient which is utilized for synthesis of ATP from ADP and inorganic phosphate.
Oxidative phosphorylation is the most common mode in all aerobically respiring organisms. In photo- phosphorylation, ATP is synthesized during photosynthetic electron transport. This type of ATP formation occurs in photosynthetic bacteria, cyanobacteria and in green plants including all types of algae.