ADVERTISEMENTS:
In this article we will discuss about the molecular structure of proteins with the help of diagrams.
Quantitatively, proteins are the chief organic components of most living organisms, as also of viruses. About 50% of the dry weight of bacterial cells is protein. The flagella, pili and fimbriae are made up entirely of proteins, and the cell membrane, cytoplasm, ribosomes of bacterial cells are also made of proteins and other organic compounds, like carbohydrates, lipids and nucleic acids. Besides these structural functions, proteins present in membrane and cytoplasm act as the major component of numerous enzymes catalyzing biochemical reactions.
Just as polysaccharides are made by linking together a large number of individual sugar molecules, so are the proteins made by binding of large number of amino acids. Such large molecules, or macromolecules, as those of polysaccharides and proteins, are called polymers.
ADVERTISEMENTS:
Because these polymers occur in biological organisms, they are better known as biopolymers. Nucleic acids are another group of important biopolymers. The individual units from which a polymer is constituted are known as monomers. In case of proteins, these monomers are amino acids.
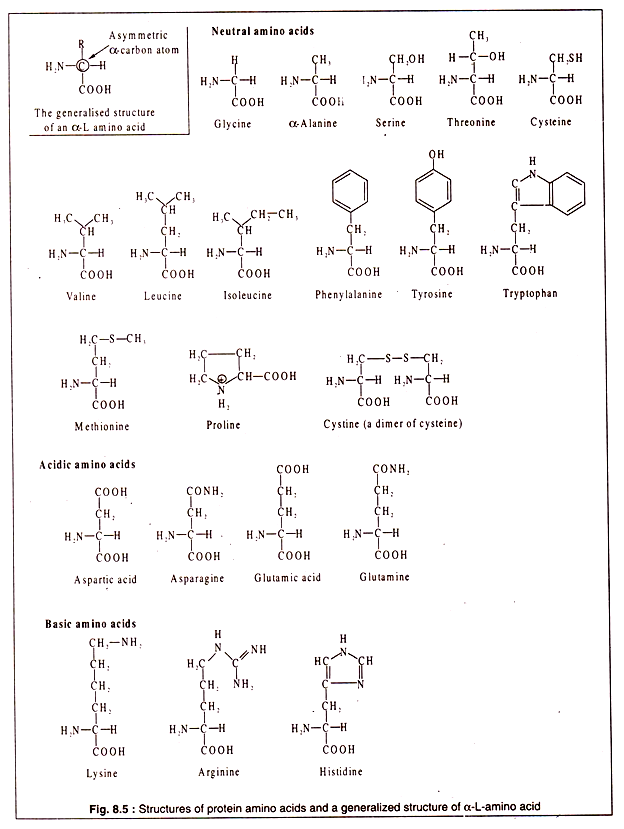
Amino acids are organic molecules containing a carboxyl (-COOH) group and an amino (-NH2) group. There are many amino acids in different groups of living organisms, but all of them are not used for synthesizing proteins. There are some 20 different amino acids which are present in most proteins. These are called protein amino acids.
All protein amino acids possess some common features. All, except one (proline), have a carbon atom to which are linked a H-atom, an amino group, a carboxyl group and a side chain (R). In one amino acid (glycine) there is a H-atom instead of R. This particular carbon atom, called the α-carbon, is asymmetric (except in glycine) and, hence, the amino acids show optical activity.
They can have a L-or D-configuration. However, the protein amino acids are all α-amino acids and have an L-configuration. As a convention, the amino group is written on the left side and the H-atom on the right side of the α-carbon atom (Fig. 8.5).
ADVERTISEMENTS:
Although all protein amino acids are α-L amino acids, some of them may possess an extra amino group or an extra carboxyl group. The presence of these additional groups makes such amino acids basic and acidic.
Thus, the protein amino acids can be divided into three broad groups:
1. Neutral,
2. Acidic and
3. Basic.
The structures of the protein amino acids are shown in Fig. 8.5:
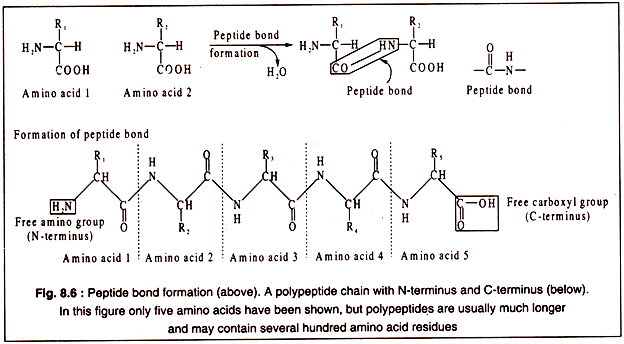
The amino acid are linked to each other by peptide bonds which are covalent in nature and involves the α-carboxyl group of one amino acid and the α-amino group of another with elimination of a water molecule as shown in Fig. 8.6. By such peptide bonds amino acids are linked to form a long chain, called a polypeptide.
Each polypeptide chain contains a free α-amino group of the initial amino acid and a free α-carboxyl group of the last amino acid of the chain. Thus, a polypeptide has two poles, an N-terminus consisting of the free α-amino group and a C-terminus having a free α-carboxyl group at the other end (Fig. 8.6).
Protein molecules are made of polypeptides. Some proteins contain a single polypeptide e.g. lysozyme, and ribonuclease. Others may have two e.g. insulin, or more e.g. haemoglobin is made of four polypeptides of two types, α and β.
Protein molecules can have four different levels of organization, known as their primary, secondary, ‘tertiary and quaternary structures. The primary structure possessed by all proteins is their specific sequence of amino acids in the polypeptide chains.
This sequence is fixed for a given polypeptide and is determined by the sequence of bases in the messenger RNA while the polypeptide is synthesized. The base sequence of the m-RNA is, in turn, a complimentary copy of the DNA strand from which it is transcribed. Thus, the amino acid sequence of a polypeptide is determined genetically. Unless there is a change in the base sequence of DNA due to a mutational event, the amino acid sequence of a polypeptide remains unaltered.
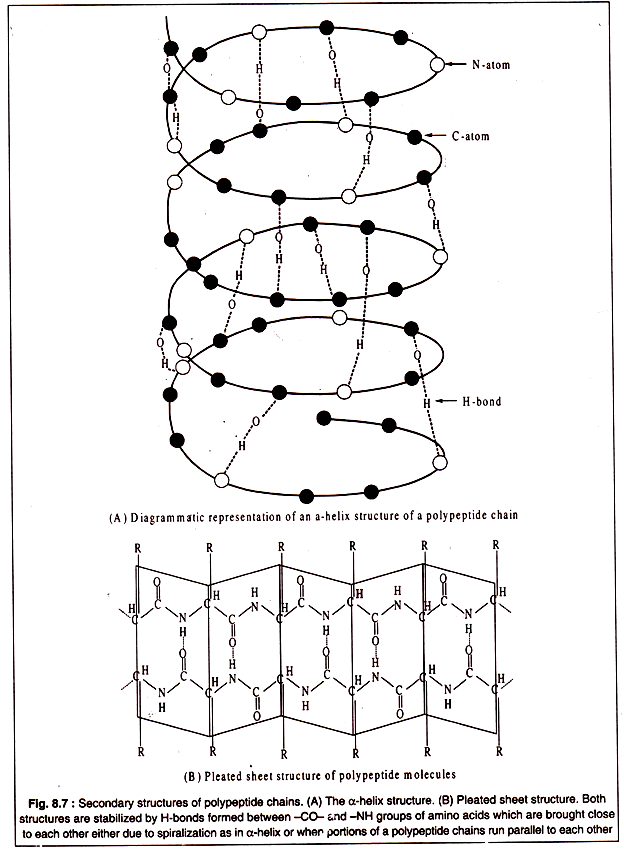
Secondary structure of polypeptides results from twisting and folding of the molecules in localized portions of the polypeptide chain. Generally, two types of folding occur. One type is formation of a clockwise spiral, called α-helix structure which is held in proper form by hydrogen bonds between the -CO-NH- groups of the constituent amino acids brought close to each other due to spiralization (Fig. 8.7A). Another type of secondary structure, known as pleated-sheet, is produced by hydrogen bonding’s between parallely running segments of a polypeptide chain (Fig. 8.7B).
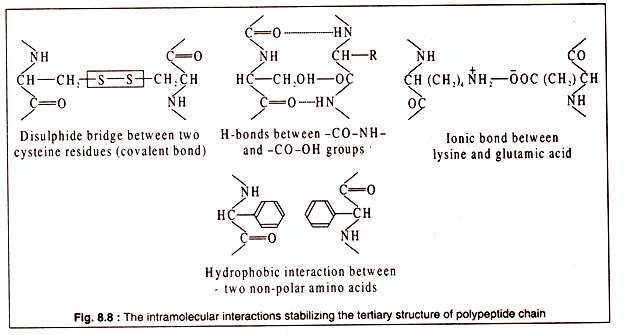
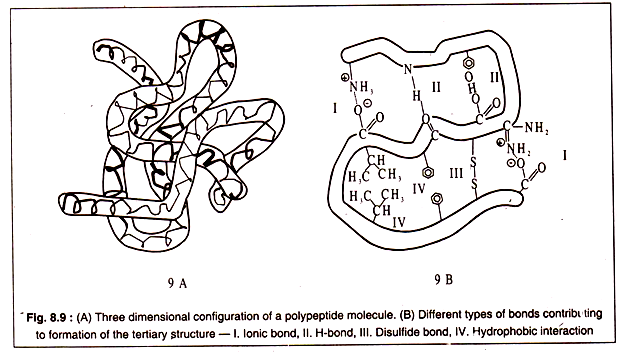
Tertiary structure of a polypeptide gives the molecule a three-dimensional shape. The polypeptide with its secondary structure further folds into a characteristic shape due to interactions of the side chains i.e. the R group of amino acids.
Interactions between the side chains may be strong due to formation of covalent bonds, like the formation of disulphide bridges between two cysteine residues, or may be weak, like the H-bonds, hydrophobic interaction or ionic bonds.
The bonds stabilizing the tertiary structure are shown in Fig. 8.8:
A three dimensional configuration of a polypeptide is diagrammatically represented in Figs. 8.9A and 9-B:
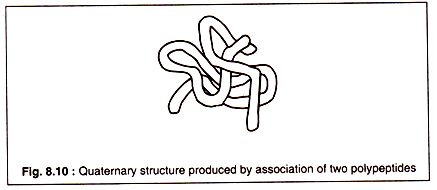
Although some proteins consist of a single polypeptide chain, many others — particularly globular proteins — having molecular weights of 60,000 Daltons or more, consist of more than one polypeptide chains. Such proteins are oligomeric and the constituent polypeptides are called protomers.
The association of protomers to form a super molecule of an oligomeric protein is known as the quaternary structure. The forces that keep the protomers together are basically the same as the intermolecular bonds which contribute to the formation of tertiary structure of individual polypeptides.
The association of two polypeptides to form a quaternary structure is shown in Fig. 8.10:
Soluble proteins subjected to abnormal conditions, such as temperature, acidity or alkalinity, high salt concentrations, solvents etc. undergo denaturation. During this process, the protein molecules lose their characteristic structures, particularly the tertiary and quaternary structures. Denatured proteins are generally biologically inactive particularly their catalytic properties are lost.
Many proteins in biological systems combine with various other organic molecules to form the conjugated proteins. Thus, when a protein combines with carbohydrates, a glycoprotein is formed. Similarly, conjugation with nucleic acids and lipids results in a nucleoprotein and lipoprotein, respectively. Combination with a chromogenic prosthetic group yields a chromo-protein such as haemoglobin.