ADVERTISEMENTS:
In this article we will discuss about the molecular structure of nucleic acid with the help of diagrams.
Nucleic acids constitute another important group of biological macromolecules present in all types of organisms where they function mainly as store-house of genetic information and as information- transfer molecules.
Two types of nucleic acids:
ADVERTISEMENTS:
i. Ribonucleic acid (RNA) and
ii. Deoxyribonucleic acid (DNA)
— exist in all cellular organisms. Viruses contain only one of these two types.
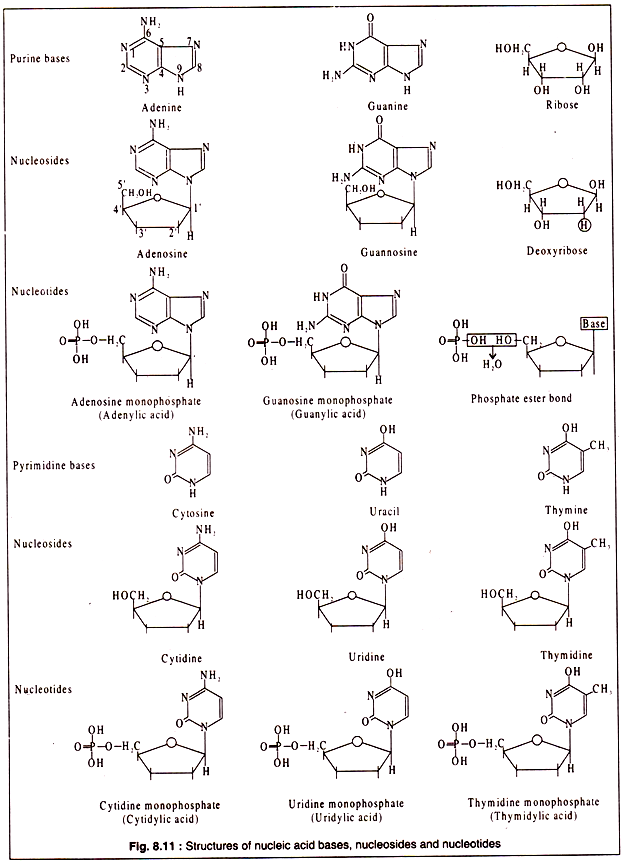
Like proteins and polysaccharides, nucleic acids also are biopolymers. The monomers are called nucleotides. Each nucleotide is again a composite molecule consisting of a pentose sugar, which is ribose in RNA and deoxyribose in DNA, a nitrogenous base and phosphoric acid.
ADVERTISEMENTS:
The nitrogenous bases are generally of five types. These are adenine, guanine, cytosine, thymine and uracil. Of these, the first three are present in both RNA and DNA, while thymine occurs in DNA and uracil in RNA.
Adenine and guanine are purines consisting of one six-membered and one five-membered ring both being heterocyclic. Cytosine, thymine and uracil are pyrimidine’s having a single six-membered heterocyclic ring. When one of these nucleic acid bases combines with a pentose sugar, a nucleoside is formed.
A combination of a nucleoside with a phosphoric acid results in a nucleotide. Nucleic acids are long chains of nucleotides.
The structures of purine and pyrimidine bases and their corresponding nucleosides and nucleotides are shown in Fig. 8.11:
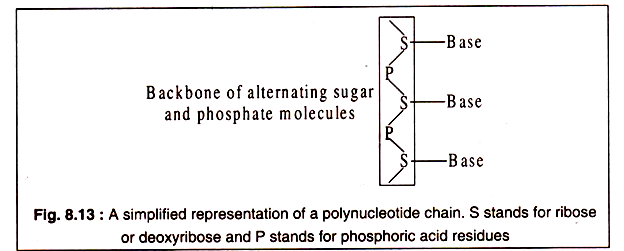
Nucleic acids are polynucleotides consisting of nucleotides linked to each other by phosphodiester bonds. Each phosphoric acid molecule forms two ester bonds, one with the 3-OH group of one ribose or deoxyribose of a nucleoside and the other with the 5′-OH group of ribose or deoxyribose of another nucleoside.
Formation of polynucleotide chain through phosphodiester bonds is shown in Fig. 8.12:
Just as a polypeptide chain has a polarity in having a free α-amino group at one end and a free carboxyl group at the other end, so has a polynucleotide chain a polarity in having a free phosphate group attached to 5/ carbon of one pentose at one end and a free (OH) group of a 3/ carbon of a pentose at the other end.
ADVERTISEMENTS:
These are generally designated as 5/ end and 3/-end of a nucleic acid molecule (Fig. 8.12). Both RNA and DNA consist of a backbone formed by alternating phosphate and pentose sugar molecules. Each sugar molecule is attached to one of the nitrogenous bases through its 1/ -carbon atom.
A simplified representation of a polynucleotide chain is shows in Fig. 8.13:
(i) Ribonucleic Acid (RNA):
Ribonucleic acids differ structurally from deoxyribonucleic acids in three respects. Firstly, the sugar component is ribose. Secondly, the nitrogenous bases are adenine, guanine, cytosine and uracil. Thirdly, RNA molecules are usually single-stranded, while those of DNA are generally double- stranded.
ADVERTISEMENTS:
There are three major types of RNA in all cellular organisms. These are ribosomal RNA (r-RNA), messenger RNA (m-RNA), and transfer RNA (t-RNA). More than 80% of an organism’s total RNA is in the form of r-RNA. Ribosomes are cytoplasmic particulate bodies consisting of RNA and protein. Ribosomes of eukaryotic and prokaryotic cells differ in size and constitution. Ribosomal RNA of prokaryotes has three components — 5S, 16S and 23S (S stands for sedimentation coefficient in Svedberg units). A prokaryotic ribosome consists of two units, a smaller 30S unit and a larger 50S unit. The intact ribosomes are 70S particles.
In bacterial cells, only about 2% of the total RNA is accounted for by m-RNA. The m-RNAs vary widely in length and in molecular weight. They have a sedimentation coefficient between 6S and 25S corresponding to molecular weights of 25,000 to 1,000,000 (1 million).
They are so named because they carry genetic information stored in DNA to the site of protein synthesis i.e. the ribosomes. The m-RNAs are synthesized by RNA polymerases by copying the sequence of bases of one of the DNA strands into a complimentary single-stranded RNA which is the m-RNA. The process of transfer of the genetic message is known as transcription.
The turnover rate of m-RNA synthesis is high, because these molecules have a short life. As soon as a polypeptide synthesis is completed, the messenger molecule breaks down to nucleotides which are then reutilized for synthesis of a new messenger molecule. On the average, synthesis of a medium-sized polypeptide takes a few minutes to be completed.
ADVERTISEMENTS:
The third type of RNA is transfer-RNA. It accounts for about 16% of total RNA in bacterial cells. Transfer-RNAs are relatively small molecules having a sedimentation coefficient of about 4S and a molecular weight of 15,000 to 30,000. Transfer RNA molecules are soluble and they occur in the cytoplasm. They contain only about 80 nucleotides.
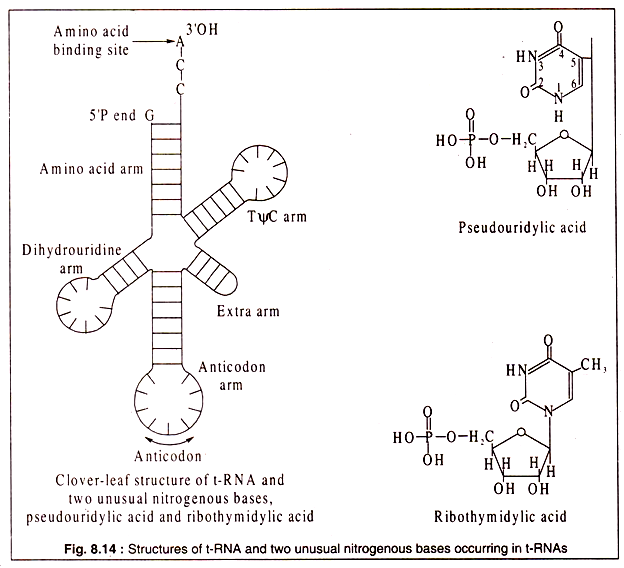
Although t-RNAs are single-stranded, the molecules fold to produce three-dimensional configurations which are commonly known as a clover leaf structure with three leaflets and a stalk (Fig. 8.14). This structure is produced by formation of double- stranded portion in certain regions where the bases are complimentary and other regions having no complimentary sequences form loops. There are many different t-RNAs in each cell.
Their function in protein synthesis is to carry the different amino acids to their correct positions on the polypeptide chain as it is being synthesized. Thus, for each of the 20 protein amino acids, there is at least one (usually more than one) specific t-RNA.
Each t-RNA contains a region with a specific sequence of three nucleotides, called the anticodon, which matches with the triplet codon of m-RNA. This assures the correct positioning of the amino acid carried by a particular t-RNA. As most of the protein amino acids are coded by more than one triplet codon (due to degeneracy of the genetic code), so there are more than 20 different types of t-RNA each having a different anticodon sequence.
Another feature of t-RNAs which is absent in r-RNA and m-RNA is the presence of unusual bases. Two common ones are pseudo-uracil and ribothymidylic acid, the structures of which have been shown in Fig. 8.14. Other unusual bases include inosine, methyl inosine, dihydrouracil etc. All t-RNAs carry a sequence of -C-C-A at the 3′-OH end which is used for binding of the specific amino acid by an ester linkage with the carboxyl group of the amino acid. Also all t-RNAs have a G-residue at the 5- P end of the molecule.
(ii) Deoxyribonucleic Acid (DNA):
In all cellular organisms, DNA consists of two polynucleotide strands forming a double-helix. Only in some DNA viruses, like bacteriophage φX174, animal virus, Parvovirus and plant virus, Gemini virus, the DNA exists naturally in single-stranded condition. In all cellular organisms and DNA- viruses, DNA acts as the genetic material.
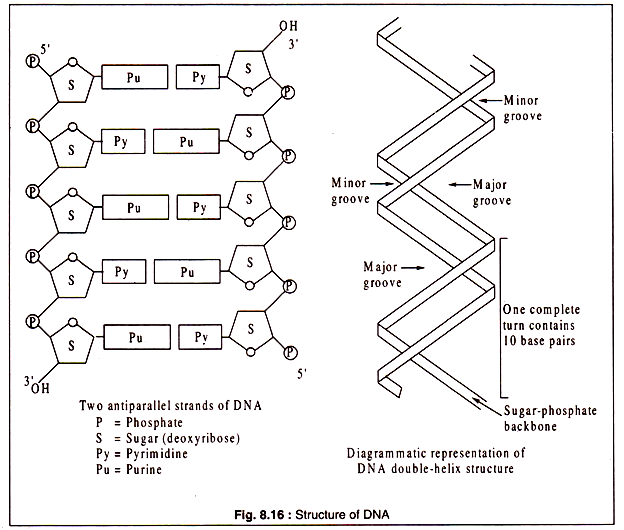
The two polynucleotide strands of a double-helix are antiparallel, i.e. the 5/ and 3/-ends of the two strands are in opposite direction. The backbone of each strand, like that of RNA, consists of alternating phosphate and sugar molecules, the sugar in DNA being deoxyribose.
The sugars are attached to the bases — adenine, guanine, cytosine and thymine through the Cl/ atom. The two polynucleotide strands form a twisted ladder-like structure of which the rungs are represented by pairs of nucleic acid bases. Each pair consists of a purine and a pyrimidine.
Chemical analysis of DNA reveals that purines (A + G) and pyrimidine’s (T + C) are always present in equimolecular proportions. This means that for every molecule of a purine base, there is one molecule of pyrimidine base. This is so, because there is complimentary base pairing in DNA. Normally, adenine always pairs with thymine by formation of two H-bonds and guanine pairs with cytosine forming three H-bonds as shown in Fig. 8.15.
ADVERTISEMENTS:
The two strands of a double-helix are held together by these H-bonds formed between the pairs of bases. When these H-bonds are disrupted, for example, by applying heat, the double helix structure collapses and the two strands separate.
A DNA sample having a higher content of G + C base pairs would naturally require a higher temperature to change from a double helix structure to single-stranded condition, because such G-C rich DNA molecules contain more H-bonds. An interesting effect of complimentary base-pairs of DNA is that the sequence of bases in one strand determines the sequence of the other strand. This has important biological implication as it would be seen later.
The double-helix structure of DNA as proposed by Watson and Crick is a right-handed (clockwise) helix containing 10 base pairs per each complete turn. The double helix structure has two grooves, one major and one minor alternating with each other. This right-handed double helix, known as the B-form of DNA appears to be the most common configuration in most organisms.
Some evidences have been produced for another configuration of DNA double-helix, known as the Z-form. The Z-form is a more compact left-handed double helix containing larger number of base pairs per turn. Diagrammatic representations of the two antiparallel strands of DNA and the double helix are shown in Fig. 8.16.