ADVERTISEMENTS:
In this article we will discuss about Electron Transport System (ETS) in living organisms.
Catabolism of energy-giving substrates (mostly glucose) up to the stage of pyruvic acid through EMP or EDP yields comparatively small amount of energy in the form of ATP by substrate-level phosphorylation. The anaerobic organisms have to be satisfied with this small amount of energy.
But the aerobic organisms can extract much larger quantity of energy from oxidation of the same amount of substrate through the TCA cycle and the electron transport system in which the H-atoms — transferred to specific acceptors, like NAD and FAD in the TCA cycle — are fully oxidized in course of electron transport.
ADVERTISEMENTS:
The ultimate acceptor of H+ and electrons is oxygen in oxygen-respiring organisms. In course of the electron transport through several carriers, ATP is generated by oxidative phosphorylation. As it will be seen, from every mole of glucose oxidized, 38 moles of ATP are formed, in contrast to only two moles in EMP and one mole in EDP.
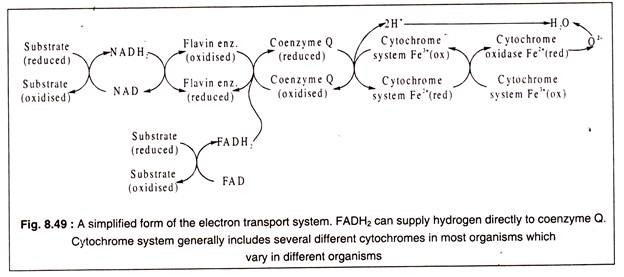
The ETS is a sequence of carrier molecules which are capable of alternately acting as an electron (or hydrogen) acceptor and electron donor, i.e. undergoing alternate reduction and oxidation. The components of ETS are located in the inner membrane of the mitochondria in eukaryotes and in the cytoplasmic membranes in prokaryotes.
There are three classes of carrier molecules. The first of these are flavoproteins which contain FMN (flavin mononucleotide) as coenzyme. An example of this class is NADH2 dehydrogenase which contains, in addition to FMN, a non-haem iron. The function of this enzyme is to transfer hydrogen from NADH2 to ubiquinone.
Ubiquinone or coenzyme Q constitutes another class of carrier molecule which can reversibly accept hydrogen atoms. It is a benzoquinone derivative with a long isoprenoid chain (R).
The third and the most important class of electron carrier molecules are constituted by the cytochromes. There are several different cytochromes, but all of them are not present in all organisms, but some members of this class are present in all aerobic organisms.
All cytochromes are composed of an iron-porphyrin prosthetic group known as haemin ring (also present in haemoglobin) attached to a protein. The haemin group is common to all cytochromes which differ from each other in their protein component. The central iron atom of the haemin ring undergoes reversible valency change with reduction or oxidation.
The terminal cytochrome of the ETS is cytochrome oxidase. It reacts with oxygen and transfers two electrons to oxygen to form doubly negatively charged oxygen atom which combines with 2H+ to form a molecule of water.
In the respiratory chain hydrogen or electrons move from a negative redox-potential to a positive one. For example, NAD/NADH2 has a redox-potential of – 0.32 volts while oxygen has a redox- potential of + 0.81 volts. During the passage from the negative to the positive potential, there is a fall in the free-energy which can be trapped for phosphorylation of ADP to form ATP. This mode of ATP formation is known as oxidative phosphorylation.
The passage of hydrogen and electrons through the ETS is shown in a simplified manner in Fig. 8.49 and in details in Fig. 8.50:
From Fig. 8.50 it may be observed that electron transport chain begins by transfer of high energy protons and electrons which have a redox potential of – 0.32 volt to gradually diminishing redox- potential. The downhill flow of electrons releases free energy which is trapped in ATP molecules.
ADVERTISEMENTS:
The production of ATP by oxidative phosphorylation of ADP can be explained by the chemiosmotic mechanism. This theory postulates that the transport of electrons by the carrier molecules which are located in the membrane creates a gradient of H+-ions across the membrane. This is possible because the membrane is impermeable to H+-ions.
The proton-gradient is formed by active transport of H+-ions by the proton pumps outwards. In case of mitochondria, H+-ion concentration increases in the space between the outer and inner membrane compared to the H+-ion concentration in the matrix. In case of bacteria, the H+-ion concentration in the surrounding medium becomes higher compared to that of the cytoplasm.
The proton gradient created by active expulsion of H+-ions by proton pumps also creates an electric charge gradient, because of accumulation of excess of positively charged H+-ions on one side of the membrane. The resulting electrochemical gradient has potential energy which is known as the proton motive force (pmf).
Protons expelled by the proton pumps can cross the membrane only through some special proton channels where an enzyme ATP synthase is located. When the protons pass through these channels the potential energy is released and it is utilized by the enzyme for synthesis of ATP from ADP and inorganic phosphoric acid. Both eukaryotic organisms and prokaryotes use the chemiosmotic mechanism for ATP synthesis.
ADVERTISEMENTS:
ATP formation by this mechanism in bacterial cells is shown diagrammatically in Fig. 8.51:
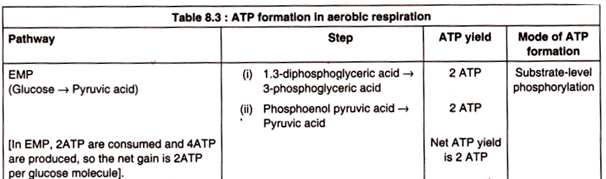
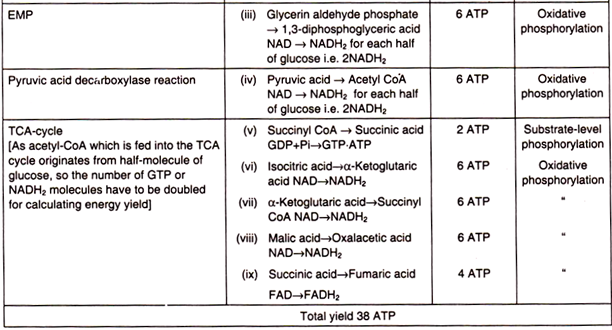
ATP yield in aerobic respiration:
The total yield of ATP when glucose is fully oxidized to CO2 and H2O amounts to 38 moles per mole of glucose. It may now be examined in which steps this amount of ATP is formed (Table 8.3). For calculating ATP yield, it should be noted that NADH2 can generate 3 ATP through ETS and FADH2 can produce 2 ATP (Fig. 8.50).
So, the overall reaction of glucose oxidation in aerobic respiration can be written as:
C6H12O6 + 6O2 + 38 ADP + 38 Pi –> 6CO 2 + 6 H2O + 38 ATP
When 1 mole of glucose is oxidized under non-biological conditions, 674 K calories of energy is liberated as heat. Now, it can be calculated how much of this energy is preserved in the form of ATP.
ADVERTISEMENTS:
Taking an average value of energy liberated by ATP hydrolysis producing ADP + Pi as -Δ K calories (ATP ADP + Pi, ∆G = -7 K cal), it is observed that a little less than 40% of the total energy released by glucose oxidation is conserved as ATP in aerobic respiration. Thus the efficiency of biological oxidation is nearly 40%. The rest of the energy is given out in the form of heat.