ADVERTISEMENTS:
The NADH, H+, and FADH2 that accumulate as the TCA cycle operates form an enormous potential energy pool.
The reduced coenzymes are reoxidized and transfer their reducing capabilities through a sequence of compounds to oxygen, which accepts electrons and H+ to form water.
The sequence of carriers through which the electrons and H + pass is called the electron transport system.
ADVERTISEMENTS:
Each transfer of electrons (or H+) from one compound to the next results, in the oxidation (electron loss) by the donor molecule and the reduction (electron gain) by the acceptor molecule.
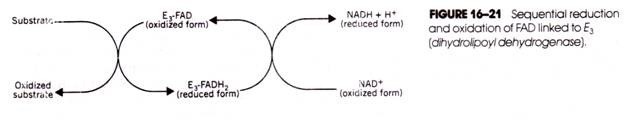
Such a transfer occurred in reaction 16-3 in the matrix during pyruvate dehydrogenase action. The enzyme-linked FAD (E3-FAD) accepted the electrons from the substrate and was reduced, thereby forming E3
FADH2. Subsequently, the E3-FADH2 transferred hydrogens and electrons to NAD+, which therefore was reduced while the FADH2 was reoxidized (Fig. 16-21).
In the electron transport system the electrons are shuttled from one to another of about 12 to 17 intermediate compounds before they eventually reach oxygen to form water. Although it is possible to directly reoxidize NADH with oxygen, in the mitochondria this is usually prevented.
ADVERTISEMENTS:
The enzymes of the electron transport system appear to be arranged in the inner membrane in such a manner that the transfer of the electrons must proceed through the specified series of intermediates. An energy change occurs during electron transfer; a proton gradient and membrane potential are ultimately created across the inner membrane. The proton gradient and membrane potential provide the energy to phosphorylate ADP—a process termed oxidative phosphorylation.