ADVERTISEMENTS:
Here is a compilation of notes on enzymes. After reading these notes you will learn about: 1. Introduction to Enzymes 2. Origin of Enzymes 3. Historical Landmarks 4. Meaning 5. Importance 6. Unit 7. Chemical Nature 8. Properties 9. Characteristics 10. Nomenclature 11. Classification 12. Enzymes Vs. Non-Biological Catalysts 13. Catalysts and Enzymes 14. Types 15. Modes of Enzyme Action and Others.
Contents:
ADVERTISEMENTS:
- Notes on Introduction to Enzymes
- Notes on the Origin of Enzymes
- Notes on the Historical Landmarks of Enzymes
- Notes on the Meaning of Enzyme
- Notes on the Importance of Enzyme
- Notes on the Unit of Enzyme
- Notes on the Chemical Nature of Enzymes
- Notes on the Properties of Enzyme
- Notes on the Characteristics of Enzymes
- Notes on the Nomenclature of Enzymes
- Notes on the Classification of Enzymes
- Notes on Enzymes Vs. Non-Biological Catalysts
- Notes on Catalysts and Enzymes
- Notes on the Types of Enzymes
- Notes on the Modes of Enzyme Action
- Notes on the Inhibition of Enzyme Action
- Notes on the Feedback Inhibition of Enzymes
- Notes on the Specificity of Enzyme
- Notes on Factors Influencing Enzyme Activities
- Notes on the Biochemical Pathways of Enzymes
- Notes on the Regulation of Enzyme
Note # 1. Introduction to Enzymes:
Thousands of chemical reactions proceed very rapidly at any given instant within all living cells of an organism. Virtually all of these reactions are mediated by remarkable molecular devices called enzymes. That is, the enzymes are central to every biochemical reaction and are called the catalysts of biological systems (biocatalysts).
ADVERTISEMENTS:
They in organized sequences and catalyse the hundreds of stepwise reactions by which nutrient molecules are degraded, chemical energy is conserved and transformed, and biological macromolecules are made from simple precursors. Through the action of regulatory enzymes, metabolic pathways are highly coordinated to yield a harmonious interplay among the many different activities necessary to sustain life.
Enzymes catalyse an enormous diversity of biochemical reactions due to their capacity to specifically bind a very wide range of molecules. By utilizing the full repertoire of intermolecular forces, enzymes bring substrates together in an optimal orientation, the prelude to making and breaking chemical bonds.
They catalyse reactions by stabilizing transition states, the highest energy-species in reaction pathways. By selectively stabilizing a transition state, an enzyme determines which one of several potential biochemical reactions actually takes place.
Until 1980s, all enzymes were believed to be proteins. Then, Tom Cech and Sidney Altman independently discovered that certain RNA molecules may function as enzymes may be effective biocatalysts. These RNA biocatalysts have come to be known as ribozymes.
Note # 2. Origin of Enzymes:
Enzymes are commonly proteinaceous substances which are capable of catalysing chemical reactions of biological origin without themselves undergoing any change. Therefore, they are called biocatalysts. Enzymes are synthesised by living cells.
The term ‘enzyme’ was coined by Kuhne (1878) for catalytically active substances previously called ferments. Enzymes were actually found out by Buchner (1897) with the accidental discovery that fermentation of sugar is not only caused by living yeast cells but also yeast extract.
The extract obviously possessed biocatalysts required for the process. Buchner (1903) also isolated the first enzyme. He was awarded Nobel Prize in the same year, 1903. There are numerous enzymes as every biochemical reaction is catalysed by a separate enzyme. It is estimated that a cell contains over 5000 chemicals. The number of chemical reactions is many times more.
Therefore, the number of enzymes is several thousands. A cell with an average diameter of 20 pm has about 1000 chemical reactions going on at any time. All of them require specific enzymes. All the enzymes are not present at all the times in the cell but they are formed as and when required from the blue print present in DNA.
ADVERTISEMENTS:
Enzymes are mainly functional inside the living cells. As found out by Buchner they can be extracted from the cells and made to catalyse reactions outside the living cells. In nature some enzymes are secreted by living cells and made to perform extracellular catalysis.
Digestive enzymes belong to this category. Several enzymes of medical and chemical importance are now available in the market, e.g., rennet tablets (from rennin of calf stomach) for coagulating milk protein casein during preparation of cheese and other milk products.
Enzymes functional outside the living cells are called exo-enzymes, e.g., enzymes present in digestive juices, lysozyme of tears. Enzymes functional inside living cells are known as endoenzymes, e.g., enzymes of Krebs cycle (inside mitochondria), enzymes of glycolysis (inside cytoplasm).
The biochemical which is acted upon by an enzyme is known as substrate. In case two bio-chemicals are involved in a reaction, the same are called reactants. The chemicals formed after the completion of a reaction are termed as products. The final products are also called end products. Part of enzyme that takes part in catalysing biochemical reaction is called active site.
ADVERTISEMENTS:
Note # 3. Historical Landmarks of Enzymes:
ADVERTISEMENTS:
The very existence of biological catalysis was first recognized and described during the late 18th century while studying the digestion of meat by secretion of the stomach. Subsequently, Louis Pasteur concluded in 1850s that fermentation of sugar into alcohol by yeast is catalyzed by “ferments”.
He postulated that these ferments were inseparable from the structure of living yeast cells, a view called ‘vitalism’ that prevailed for many years. F.W. Kuhne coined the term enzyme in 1878 to represent the “ferments”. The first enzyme was isolated by E. Buchner in 1903 for which he was awarded Nobel Prize in the same year.
ADVERTISEMENTS:
The protein nature of enzyme was first discovered by James Sumner in 1926 when he purified the enzyme urease and obtained it in crystalline form. For this, Sumner was awarded Nobel Prize in 1946.
Note # 4. Meaning of Enzyme:
An enzyme is a protein that is synthesised in a living cell and catalyses or speeds up a thermodynamically possible reaction so that the rate of the reaction is compatible with the biochemical process essential for the maintenance of the cell. It is sometimes called as organic catalyst or biocatalyst.
ADVERTISEMENTS:
Over 90% of enzymes are simple globular proteins (Fig. 8.14). The remainder is conjugated proteins, which have a non-protein fraction called the prosthetic group. Many enzymes have relative molecular mass of between 10,000 and 50,000da.
The first enzyme discovered was amylase, which catalyses the conversion of starch to maltose, in 1833 by two French chemists Payen and Persoz. However, it was not well-known until 1876 when Wilhelm Kuhne, the distinguished German biochemist, proposed the term enzyme.
Note # 5. Importance of Enzyme:
Biological Importance of Enzymes:
ADVERTISEMENTS:
(i) Thousands of chemical reactions are taking place in the body of a living organism. All of them are mediated by enzymes,
(ii) Enzymes are specialised catalysts that operate at biological temperatures,
(iii) Enzyme mediated reactions do not require harsh treatment,
(iv) They are pH specific so that reactions requiring different pH operate in different parts of the body,
(v) As they operate under favourable conditions, enzymes force the organisms to live under favourable environment,
ADVERTISEMENTS:
(vi) Enzymes are highly regulated. Their formation is controlled by separate genes. Activation and repression of genes allow certain enzymes to be functional or non-functional in cells.
Economic Importance of Enzymes:
i. ELISA:
It is enzyme based detection of diseases like AIDS.
ii. Endonucleases:
They are enzymes used in breaking DNA at specific sites. DNA fragments are employed in genetic engineering.
iii. Alcoholic Beverages:
Enzyme complex zymase obtained from yeast is used in brewing or fermentation of alcoholic drinks.
iv. Detergents:
They contain protease for brighter washing of clothes and amylase for dish washing.
v. Baby Foods:
Trypsin is added to partially pre-digest baby foods.
vi. Streptokinase:
The enzyme is used in clearing blood clots inside blood vessels.
vii. Digestive Aids:
Diastase and other enzymes are used regularly by patients with deficient digestive juices.
viii. Cheese:
Rennet or rennin tablets are used for preparation of cheese. Lactase and lipase are employed to provide proper consistency and flavour to cheese.
ix. Pectinase:
It is used for clearing fruit juices, retting of fibres and preparation of green coffee.
x. Protease:
The enzyme is employed for chill-proofing of beverages, degumming of silk, cleaning of hides, softening of bread and meat.
Note # 6. Unit of Enzyme:
The actual molar amount of the enzyme in an enzyme-catalyzed reaction is not known in many situations. In such cases the amount of enzyme can be expressed in terms of the enzyme activity observed.
The International Commission on Enzymes established by International Union of Biochemistry defines One International Unit of enzyme as the amount of enzyme that catalyzes the formation of one micromole of product in one minute.
In determining the One International Unit the conditions of assay must be specified because enzymes are very sensitive to factors such as pH, temperature, and ionic strength. Another definition for units of enzyme is the ‘katal’. One katal is defined as the amount of enzyme that catalyses the conversion of one mole of substrate to product in one second. Thus, one katal equals 6 x 107 international units.
Note # 7. Chemical Nature of Enzymes:
All enzymes are globular proteins with the exception of recently discovered RNA enzymes. Some enzymes may additionally contain a non-protein group. Accordingly there are two types of enzymes, simple and conjugate.
Simple Enzyme:
It is an enzyme which is wholly made up of protein. Active site is formed by specific grouping of its own amino acids. Additional substance or group is absent, e.g., pepsin, trypsin, urease.
Conjugate Enzyme:
It is an enzyme which is formed of two parts— a protein part called apoenzyme (e.g., flavoprotein) and a nonprotein part named cofactor. The complete conjugate enzyme, consisting of an apoenzyme and a cofactor, is called holoenzyme. Active site is formed jointly by apoenzyme and cofactor.
Cofactor is small, heat stable and dialysable part of conjugate enzyme. It may be inorganic or organic in nature. Organic cofactors are of two types, coenzymes and prosthetic groups.
Coenzymes are easily separable non-protein organic cofactors. Prosthetic groups are non-protein organic cofactors firmly attached to apoenzymes, e.g., heme (=haem), biotin, pyridoxal phosphate. Heme (= haem) is iron containing prosthetic group in cytochromes, haemoglobin, myoglobin, catalase and peroxidase.
The last two cause breakdown of hydrogen peroxide to water and oxygen. FMN and FAD are considered prosthetic groups by some workers while others consider them to be coenzymes.
Both coenzyme and prosthetic group take part in group transfer reactions. Prosthetic group requires a single apoenzyme for picking up the group and transferring the same. Coenzyme requires two Apo enzymes, one for picking up the group and the second for transferring the group, e.g., NAD+, NADP+, CoA.
Coenzyme has three important functions:
(a) Coenzyme is essential for bringing the substrate in contact with the enzyme,
(b) It picks up a product of the reaction, e.g., hydrogen in case of NAD+ (nicotinamide adenine dinucleotide) or NADP+.
(c) The product picked up by a coenzyme is transferred to another reactant.
Certain workers use the term cofactor for any loosely bound non-protein group. The organic cofactor is called coenzyme. They use the term prosthetic group similarly for both inorganic and organic group attached firmly to apoenzyme.
Most of the coenzymes are made of water soluble vitamins, В and C, e.g., thiamine, riboflavin, nicotinamide, pyridoxine. Inorganic cofactors include ions of a variety of minerals e.g., calcium, iron, copper, zinc, magnesium, manganese, potassium, nickel, molybdenum, selenium, cobalt.
They usually function as activators by forming one or more coordination bonds with both the substrate and active site of enzyme. Fe2+ is cofactor for catalase. Chloride ion stimulates activity of salivary amylase. Zinc is required for carboxypeptidase NAD+ and NADP+ activity.
Active Site or Active Spot:
The whole of enzyme molecule is not active in catalysing a chemical reaction. Only a small portion of it is active. It is called active site or active spot. An enzyme may have one to several active sites. An active site or spot is an area of the enzyme which is capable of attracting and holding particular substrate molecules by its specific charge, size and shape so as to allow the chemical change.
It fails to recognise other molecules. Active site consists of a few amino acids and their side groups which are brought together in a particular fashion due to secondary and tertiary folding of a protein molecule (Fig. 9.27) and its association with the cofactor, if any.
For example, the active site for aldolase is glycine-histidine-alanine while that of pyruvic oxidase is aspartic acid-cysteine-alanine. The remaining amino acids help maintain the shape of the enzyme molecule.
Note # 8. Properties of Enzyme:
Some of the properties of enzyme are as follows:
i. Protein Nature:
Enzymes are generally globular proteins. They may have additional inorganic or organic substances for their activity. However, two types of RNA enzymes are known, ribozyme and ribonuclease-P. Peptidyl transferase has also been found to be part of rRNA by Noller (1992).
ii. Molecular Weight:
Being proteinaceous, the enzymes are giant molecules with a molecular weight of 6000 (bacterial ferredoxin) to 4,600,000 (pyruvate dehydrogenase complex).
iii. Colloidal Nature:
They are hydrophilic and form hydrosol in the Free State.
iv. Chemical Reaction:
Enzymes do not start a chemical reaction but increase the rate of chemical reaction. They do not change the equilibrium but bring about equilibrium very soon.
v. Efficiency:
The number of substrate molecules changed per minute by a molecule or enzyme is called turn over number (kcat). The higher the turn-over number, the more efficient an enzyme is. It depends upon the number of active points present over an enzyme, precise collisions between reactants and the rate of removal of end products.
The optimum turn-over number for enzyme carbonic anhydrase (enzyme present in RBCs) is 36 million, catalase 5 million, enzyme sucrase or invertase 10,000 and flavoprotein 50. Enzyme efficiency is usually much more than that of inorganic catalysts.
For example:
The rate of enzyme mediated hydrolysis of urea is 1014 times higher than the rate of its acid hydrolysis carried out at 40°C higher temperature. Similarly, the rate of CO2 hydration is 10 million times faster in the presence of enzyme carbonic anhydrase than in its absence.
vi. Unchanged Form:
Enzymes are in no way transformed or used up in the chemical reaction but come out unchanged at the end of reaction.
vii. Reversibility:
Theoretically, all enzyme controlled reactions are reversible. Reversibility is, however, dependent upon energy requirements, availability of reactants, concentration of end products and pH.
viii. Enzyme Specificity:
Enzymes are highly specific in their action. For example, enzyme maltase acts on sugar maltose but not on lactose or sucrose. Different enzymes may act on the same substrate but give rise to different products.
For example, raffinose gives rise to rnelibiose and fructose in the presence of enzyme sucrase while in the presence of enzyme melibiase it produces lactose and sucrose. Similarly an enzyme may act on different substrates, e.g., sucrase can act on both sucrose and raffinose producing different end products.
ix. Heat Sensitivity:
All enzymes are heat sensitive or thermolabile. Most enzymes operate optimally between 25°-35°C. They become inactive at freezing temperatures and denatured at 50°-55°C. However, thermal algae and bacteria are an exception. Their enzymes remain functional even at 80°C. Enzymes of seeds and spores are also not denatured at 60°— 70°C.
x. Protein Poisons:
Being made of proteins, enzymes are inactivated or denatured by all those substances and forces which destroy protein structure, e.g., heavy metals, high energy radiations.
xi. pH:
Each enzyme functions at a particular pH (Fig. 9.28.), e.g., pepsin (2 pH), sucrase (4.5 pH), salivary amylase (6.8 pH), trypsin (8.5 pH). A change in pH makes the enzymes ineffective.
Specificity of pH for enzyme activity is useful in regulating enzymes, e.g., salivary amylase stops its activity in stomach where hydrochloric acid is secreted. The same acid activates another enzyme pepsin from its precursor called pepsinogen. Pepsinogen can also be changed into pepsin by catalytic activity of the latter.
xii. Enzyme-Substrate Complex:
The active sites of enzymes have a specific conformation for attracting and holding substrate. It usually possesses a crevice or pocket where the substrate fits in a complementary fashion. The two join to form a complex known as enzyme—substrate complex (ES).
The complexed state is short lived. The substrate is changed into products. The products remain complexed with the active site of the enzyme for a brief period. They soon separate and the active site is freed to perform another catalytic act.
The greater the affinity of the enzyme for a substrate, the higher is the catalytic activity.
xiii. Chain Reactions:
Biochemical reactions are not isolated. A number of them occur in quick succession. A team of enzymes work one after the other to accomplish such multistep reactions, e.g., five enzymes for conversion of threonine to isoleucine.
Note # 9. Characteristics of Enzymes:
All enzymes are proteins, but a functional enzyme has different components and these components are named differently, viz.,
Holoenzyme:
A conjugated protein and functional enzyme.
Apoenzyme:
Polypeptide segment of the enzyme, which is catalytically inactive.
Coenzyme:
The non-protein organic moiety, which can frequently be separated from the apoenzyme.
Prosthetic group:
If a substance is firmly (covalently) attached to the protein part of the enzyme, it is referred to as a prosthetic group. It is the non-protein portion of any conjugated protein. So coenzyme is a specific example of prosthetic group.
Activator:
There are many metalloprotein enzymes in which the metal ion (e.g. Mg++, Mn++, and Zn++) is bonded either to the apoenzyme or to the coenzyme. The metal is usually designated as activator. They form a co-ordination complex between the enzyme and the substrate, and activate the substrate by prompting electronic shifts.
Pro-enzyme or Zymogens:
They are simple protein enzymes, which are secreted, in an inactive form.
Activation:
It is the process in which an inactive protein (pro-enzyme or zymogens) is transformed into an active enzyme.
Note # 10. Nomenclature of Enzymes:
All enzyme names should end in suffixase. Exceptions are some old names, e.g., ptyalin, pepsin, trypsin. Some old names indicate the source but not the action, e.g., papain from Papaya, bromelain from Pineapple of family Bromeliaceous.
In modem system enzyme names are given after:
(i) Substrate acted upon, e.g., sucrase (after sucrose), lipase, proteinase, nuclease, peptidases, maltase
(ii) Chemical reaction, e.g., dehydrogenase, oxidase, carboxylase, decarboxylase, etc.
The second category of names are group names. They are often qualified by the addition of the name of substrate, e.g., succinic dehydrogenase, isocitric dehydrogenase, glutamate-pyruvate transaminase, DNA polymerase.
Thus DNA polymerase catalyses synthesis of DNA segments through polymerisation of deoxyribonucleotides. Similarly glutamate-pyruvate transaminase transfers amino group (—NH2) from glutamate to pyruvate.
Note # 11. Classification of Enzymes:
In older times enzymes were classified into two broad categories:
(i) Hydrolysing:
Catalysing hydrolysis of larger molecules into smaller ones, e.g., carbohydrates or amylases, proteases, lipases, esterases, phosphorylases, amidases. Digestive enzymes are hydrolysing in nature. They are often grouped into three types— proteolytic, amylolytic and lipolytic,
(ii) Desmolysing:
Catalysing reactions other than hyrolysis, e.g., aldolases, dehydrogenases, oxidases, peroxidases, catalases, carboxylases, etc. The modem system of enzyme classification was introduced by International Union of Biochemistry (IUB) in 1961. It groups enzymes into the following six categories.
a. Oxidoreductases:
They take part in oxidation and reduction reactions or transfer of electrons.
Oxidoreductases are of three types— oxidases, dehydrogenases and reductases, e.g., cytochrome oxidase (oxidises cytochrome), succinate dehydrogenase, nitrate reductase.
b. Transferases:
They transfer a group from one molecule to another e.g., glutamate- pyruvate transaminase (transfers amino group from glutamate to pyruvate during synthesis of alanine). The chemical group transfer does not occur in the Free State.
c. Hydrolases:
They catalyse hydrolysis of bonds like ester, ether, peptide, glycosidic, С-С, С halide, P—N, etc. which are formed by dehydration condensation. Hydrolases break up large molecules into smaller ones with the help of hydrogen and hydroxyl groups of water molecules. The phenomenon is called hydrolysis. Digestive enzymes belong to this group, e.g., amylase (hydrolysis of starch), sucrase, lactase.
d. Lyases:
The enzymes cause cleavage, removal of groups without hydrolysis, addition of groups to double bonds or removal of a group producing double bond, e.g., histidine decarboxylase (breaks histidine to histamine and CO2), aldolase (fructose-1, 6-diphosphate to dihydroxy acetone phosphate and glyceraldehyde phosphate).
Fructose 1, 6-diphosphate – aldolase → Dihydroxy acetone phosphate + Glyceraldehyde phosphate.
e. Isomerases:
The enzymes cause rearrangement of molecular structure to effect isomeric changes. They are of three types, isomerases (aldose to ketose group or vice-versa like glucose 6-phosphate to fructose 6-phosphate), epimerases (change in position of one constituent or carbon group like xylulose phosphate to ribulose phosphate) and mutases (shifting the position of side group like glucose-6-phosphate to glucose-1- phosphate).
f. Ligases (Synthetizes):
The enzymes catalyse bonding of two chemicals with the help of energy obtained from ATP resulting in formation of such bonds as С-О, С-S, С-N and P-O, e.g., pyruvate carboxylase. It combines pyruvic acid with CO2 to produce oxaloacetic acid.
Activation Energy:
Most of the chemical reactions do not start automatically because the reactant molecules have an energy barrier to become reactive.
The energy barrier may be on account of:
(i) Mutual repulsion due to presence of electrons over their surfaces,
(ii) Solvation or holding of reactants in solution form by hydrogen bonds,
(iii) Reaction sites of the reactive molecules being small, precise collisions do not occur.
Therefore, an external supply of energy is needed for the start of the chemical reaction. It is called activation energy. Activation energy increases the kinetic energy of the system and brings about forceful collisions between the reactants. The requirements of activation energy is quite high. For example, acidic hydrolysis of sucrose requires 32000 cal/ mole of energy.
As already noted about 1000 chemical reactions are taking place in a cell at any time. Activation energy required for such a large number of reactions cannot be provided by living systems. Enzymes lower the activation energy required for a reaction (Fig. 9.32).
For example, in the presence of enzyme sucrase or invertase, hydrolysis of sucrose requires an activation energy of 9000 cal/mole (instead of 32,000 cal/ mole).
This is achieved by four ways:
(i) De-solvation or taking the reactants out of solution state,
(ii) Establishing weak bonds between reactants and enzyme. It releases energy called bond energy,
(iii) Bringing the reactant molecules closer in the region of active sites of enzymes,
(iv) Development of strain in the bonds of the reactants by electrophilic and nucleophilic attack,
(v) Formation of unstable intermediate structural states collectively called transition state. During the transition state the substrate bonds are broken and new bonds are established that transform the substrate molecules into products,
(vi) In exothermic reactions, the energy content of the products is lower than that of substrate (Fig. 9.32).
It is higher in case of endothermic reactions. However, whether the reaction is endothermic or exothermic, energy is required for pushing the substrate molecules into transition state. The difference in the energy level of substrate (S) and transition state is the activation energy required to start the reaction.
Note # 12. Enzymes Vs. Non-Biological Catalysts:
A catalyst is a molecule that accelerates a particular chemical reaction without itself being chemically altered in the process, and may be biological or non-biological in origin. Biological catalysts are the enzymes.
Enzymes are similar to non-biological catalysts in the following respects:
(i) They lower the activation energy of reaction,
(ii) They do not participate in the reaction, and return in their original form at the end of reaction, and
(iii) They only increase the reaction rate.
But enzymes increase the rate of reaction at a phenomenal scale, and are highly specific. These features are unimaginable for non-biological catalysts (Table 27.1). Many enzymes may be less specific in binding to the substrate, but they are always extremely specific in the reaction they catalyze. For example, mammalian cytochrome P450 binds to a variety of substrates, but it always adds a -OH group to the substrate.
But many enzymes are highly specific in binding as well, e.g., glucose oxidase binds only to D- glucose. Enzymes can distinguish between similar parts of the substrate molecule (this property is called regiospecificity) and between optical isomers of the substrate (this ability is called stereo-specificity).
In addition, enzymes are subject to a variety of regulations, and their reaction rates show substrate saturation, which is not the case with catalysis (non-biological).
Enzymes are attractive because they operate under mild conditions of temperature, pressure and pH, which saves energy, and undesirable by-products are not produced by enzymes; this simplifies product recovery. Finally, certain stereo-chemical reactions are impractical with chemical methods.
The disadvantages associated with enzymes are as follows:
(i) High costs of enzymes, and
(ii) General instability of purified enzymes so much so that some enzymes cannot be used due to instability.
Note # 13. Catalysts and Enzymes:
Catalysts are inorganic substances which increase the rate of chemical reactions without themselves undergoing any change and without modifying the equilibrium of the reactions. Enzymes are similar chemicals which are biological in origin and operate in the biochemical world.
Similarities:
i. Unchanged:
Both catalysts and enzymes remain unchanged chemically and quantitatively at the end of the reaction, so that they can be used over and over again.
ii. Quantity:
They are required in minute quantity as compared to their substrate.
iii. Reversibility:
Reactions controlled by both catalysts and enzymes are theoretically reversible though reversibility is dependent upon different kinetics.
iv. Equilibrium:
They do not change the equilibrium of the reaction.
v. Reaction Velocity:
Both catalysts and enzymes increase the rate of chemical reaction. They do not initiate the reaction.
vi. Activation Energy:
They lower the activation energy required for starting the chemical reaction.
vii. Complexes:
They form short lived complexes with the substrate molecules.
viii. End Products:
End products of a reaction are not changed by catalysts and enzymes.
Note # 14. Types of Enzymes:
Enzymes are of three types:
i. Pro-Enzyme or Zymogen,
ii. Allosteric Enzymes,
iii. Isoenzymes (Isozymes)
i. Pro-Enzyme or Zymogen:
Pro-enzyme is the inactive precursor of an enzyme. The term zymogen is often used for inactive precursor of proteolysis enzyme, e.g., pepsinogen for enzyme pepsin. Many enzymes are initially produced in the pro-enzyme or zymogen state.
They become reactive or active enzymes only at a particular pH, in the presence of substrate or some special treatment. For example, pepsinogen is changed to active enzyme pepsin in the presence of hydrochloric acid of gastric juice. Thereafter, pepsin has autocatalytic effect on further conversion of pepsinogen.
ii. Allosteric Enzymes:
They are enzymes which have separate areas for different types of modulators that alter the conformation of the active site so as to make it effective or ineffective (Fig. 9.36). The areas are called allosteric sites. The substances which cause change in allosteric sites are known as modulators, allosteric substances or effectors.
The latter are of two types— activators and inhibitors. Allosteric activator binds with an allosteric site in such a way as to make active site operational. Allosteric inhibitor, on the other hand, brings about such a change in the active site that it becomes unable to combine with substrate molecules. For example, the enzyme phosphofructokinase is activated by ADP and inhibited by ATP.
iii. Isoenzymes (Isozymes):
At one time it was believed that an organism has only a single enzyme for a given step of a metabolic reaction. It was later discovered that a substrate may be acted upon by a number of variants of an enzyme producing the same product.
The multiple molecular forms of an enzyme occurring in the same organism and having a similar substrate activity are called isoenzymes or isozymes. Over 100 enzymes are known to have isoenzymes.
Thus a- amylase of wheat endosperm has 16 isozymes, lactic dehydrogenase has 5 isoenzymes in man, while alcohol dehydrogenase has 4 isozymes in maize. Isoenzymes differ in activity optima and inhibition. They are thus useful to organism in adapting to varied environmental conditions.
Note # 15. Modes of Enzyme Action:
There are two view points by which enzymes are supposed to bring about chemical reaction.
i. Lock and Key Hypothesis:
It was put forward by Emil Fischer in 1894. According to this hypothesis, both enzyme and substrate molecules have specific geometrical shapes. ‘In the region of active sites the surface configuration of the enzyme is such as to allow the particular substrate molecules to be held over it. The active sites also contain special groups having —NH2, —COOH, —SH for establishing contact with the substrate molecules.
The contact is such that the substrate molecules or reactants come together causing the chemical change. It is similar to the system or lock and key. Just as a lock can be opened by its specific key, a substrate molecule can be acted upon by a particular enzyme. This also explains the specificity of enzyme action.
After coming in contact with the active site of the enzyme, the substrate molecules or reactants form a complex called enzyme-substrate complex. In the complexed state the molecules of the substrate undergo chemical change.
The products remain attached to the enzyme for some time so that an enzyme-product complex is also formed. However, the products are soon released (Fig. 9.34) and the freed enzyme is able to bind more substrate molecules.
Enzyme + Substrate ⇋ Enzyme – Substrate Complex
Enzyme – Substrate Complex ⇋ Enzyme – Products Complex
Enzyme – Products Complex ⇋ Enzyme + Products
Thus we see that the chemical reactants do not cause any alteration in the composition or physiology of the enzyme. The same enzyme molecule can be used again and again (Fig. 9.35). Hence, enzymes are required in very small concentrations.
Evidences:
1. Blow and Steitz (1970) have found the formation of complex between the enzyme chymotrypsin and its substrate.
2. Keilen and Maun have observed that the absorption spectra of the same enzyme are different in the free state and in the presence of the substrate.
3. The theory explains how a small concentration of enzyme can act upon a large amount of the substrate.
4. Lock and key theory explains how the enzyme remains unaffected at the end of chemical reaction.
5. It is able to predict the increase in the rate of chemical reaction on the addition of more enzyme or substrate.
6. The theory explains how a substance having a structure similar to the substrate can work as competitive inhibitor.
ii. Induced-Fit Theory (Fig. 9.35):
It is modification of lock and key hypothesis which was proposed by Koshland in 1959. According to this theory the active site of the enzyme contains two groups, buttressing and catalytic. The buttressing group is meant for supporting the substrate. The catalytic group is able to weaken the bonds of reactants by electrophilic and nucleophilic forces.
The two groups are normally at a distance. As soon as the substrate comes in contact with the buttressing group, the active site of the enzyme undergoes conformational changes so as to bring the catalytic group opposite the substrate bonds to be broken.
Catalytic group helps in bringing about chemical reaction. The substrate is converted into product. The product is unable to hold on the buttressing site due to change in its structure and bonds. Buttressing group reverts to its original position. The product is released.
Note # 16. Inhibition of Enzyme Action:
Reduction or stoppage of enzyme activity due to presence of adverse conditions or chemicals is called enzyme inhibition. It is of several types. Inhibition can be classified into two (a) Reversible and irreversible (b) Competitive and non-competitive.
Reversible inhibition is that inhibition which can be overcome by withdrawal of the inhibitor because the effect of the latter is of temporary nature due to blocking of active site or binding to linkages required for maintenance of active site. Dilution and dialysis reduces or eliminates the effect of reversible inhibition. Irreversible inhibition is of permanent nature as the enzyme conformation is harmed.
Denaturation of enzyme is an example of irreversible inhibition. Heavy metals (e.g., Ag+, Hg2+, As+) and iodoacetic acid cause irreversible inhibition by combining with —SH groups and destroying protein structure. Dilution and dialysis have little effect once irreversible inhibition has set in.
Competitive inhibition is caused by swamping of the active sites by a chemical which is similar in structure to the substrate but does not undergo chemical change. Competitive inhibition is usually reversible. Non-competitive inhibition is caused by alteration of conformation of the enzyme by a chemical that binds to a site other than the active site. It may be reversible or irreversible.
Four common types of enzymes inhibition are as follows:
i. Protein Denaturation:
Enzyme activity is dependent upon the maintenance of tertiary structure of the protein moiety. The latter is destroyed by several factors like heat, high energy radiations and salts of heavy metals.
ii. Competitive inhibition:
It is the inhibition of enzyme activity by the presence of a chemical that competes with the substrate for binding to the active site of the enzyme. The inhibitor chemical is also called substrate analogue or competitive inhibitor.
It resembles the substrate in structure and gets bound up to the active site of the enzyme without getting transformed by the latter (Fig. 9.37). As a result, the enzyme cannot participate in catalytic change of the substrate. This is similar to the jamming of a lock by a key similar to original one.
Equilibrium constant for inhibitor binding is called Ki. A high Ki reduces enzyme activity while a low Ki allows enzyme activity to continue though at a reduced rate. Classical example of competitive inhibition is reduction of activity of succinate dehydrogenase by malonate, oxaloacetate and other anions which resemble succinate in their structure.
Competitive inhibition is usually reversible since the addition of more substrate tends to reduce the effect of the inhibitor.
The inhibition is important in that:
(i) It gives evidence for lock and key hypothesis of enzyme action,
(ii) Substrate analogues are not metabolized by enzymes,
(iii) Control of bacterial pathogens has been effected through competitive inhibition.
Sulpha drugs (e.g., sulphanilamide) inhibit the synthesis of folic acid in bacteria by competing with p-amino benzoic acid (PABA) for the active site of enzyme. Preformed folic acid is obtained by animal cells. Therefore, sulpha drugs do not harm them.
iii. Non-competitive Inhibition:
It is an irreversible inhibition of enzyme activity by the presence of a substance that has no structural similarity with the substrate. It is of two types, reversible and irreversible.
The irreversible non-competitive inhibitor destroys or combines irreversibility with a functional group of enzyme that is essential for its catalytic function. Cyanide inhibits the activity of cytochrome oxidase by combining with its metallic ions.
It has no structural similarity with the substrate of the enzyme, namely cytochrome c. Cytochrome oxidase is a respiratory enzyme. In its inhibition, the animal is unable to perform the respiration properly and gets killed. Di-isopropyl fluorophosphates (DFP, a nerve gas) prevents impulse transfer by combining irreversibly with amino acid serine of acetylcholine esterase.
It also poisons a number of other enzymes like trypsin, chymotrypsin, phosphoglucomutase, elastase, etc. lodoacetamide inhibits enzymes having sulphahydryl (—SH) or imidazole group.
iv. Allosteric Modulation or Feed Back Inhibition:
It is a type of reversible inhibition found in allosteric enzymes. The inhibitor is non-competitive and is usually a low molecular intermediate or product of a metabolic pathway having a chain of reactions involving a number of enzymes. It is, therefore, also called end product or feedback inhibition.
The inhibitor is also called modulator. Modulator is a substance that attaches with an allosteric enzyme at a site other than catalytic one but influences the latter, either inhibiting or activating the same. An example of feed back or allosteric inhibition is stoppage of activity of enzyme hexokinase (glucokinase) by glucose-6-phosphate, the product of reaction catalysed by it (Fig. 9.38).
Another example is inhibition of threonine deaminase by isoleucine (Fig. 9.3). Amino acid isoleucine is formed in bacterium Escherichia coli in a 5-step reaction from threonine. Each step requires a separate enzyme. When isoleucine accumulates beyond a threshold value, its further production stops.
Isoleucine added to the medium of bacterium also stops its internal production showing that its excess prevents some step of the reaction. The latter was found out to be enzyme threonine deaminase which is involved in the first step of the reaction (threonine to a-ketobutyrate).
Importance:
(i) It has a regulatory role on enzyme activity,
(ii) Enzyme inhibitors have been used in the study of metabolic pathways,
(iii) Some inhibitors are used in controlling pathogenic activity, e.g., sulpha drugs,
(iv) Use of inhibitors have shown the mechanism of enzyme action.
Qualitative feast for carbohydrates/Sugar:
(a) Tests for Glucouse and Fructose:
1. Grape Juice/Fruit Juice contains glucose and fructose. Their presence can be tested by Fehling’s test. Take 5 ml of fruit juice in a test tube. Add an equal quantity of Fehling solution I and II. Boil. A brick red precipitate of cuprous oxide indicates the presence of glucose or fructose in fruit juice. (Cu+2 (Blue) → Cu+red)
2. Fructose gives a red colour with Selivenoff’s reagent (resorcinol + conc. HCI) while glucose gives no colouration.
3. Glucose chars only on heating by the action of conc. H2SO4 while fructose chars in cold.
4. To 5 cc of Benedict’s solution, add 3cc of glucose solution. On boiling, green/red yellow/rust brown precipitate is formed.
5. Sucrose gives negative with Fehling’s test. It is first hydrolysed by conc. H2SO4. Boil 5cc of sucrose solution with few drops of conc. H2SO4. It is then neutralized with NaOH. Boil again and then add Fehling’s solution I and II drop by drop. A brick red precipitate is formed due to hydrolysis of sucrose into glucose and fructose by H2SO4.
6. Stain the pulp of apple/melon with methylene blue. Pectic substances in cell wall are stained violet.
(b) Tests for fats and oils/lipids:
1. Fats are insoluble in water but soluble in ether/acetone/benzene.
2. To few ml of castor oil, add few drops of Sudan III solution. A reddish colour appears.
3. Dissolve fat in alcohol and add a few drops of distilled water. An emulsion of fat in water appears on the surface. Now add few drops of 0.1% sudan III solution made in alcohol. The emulsion turns red.
4. Take 50 ml of fat sample. Add to it 100 ml of 10% NaOH. Boil for 30 minutes. Divide it into two parts A and B. To A part, add few drops of conc. H2SO4. A soapy layer collects at the surface. To В part add saturated solution of NaCI gradually. Soap precipitates and rises to the surface.
5. Test for glycerol: Mix 1 ml of 1% CuS04 solution and 5 drops of the glycerol. To it add 5 drops of 10% NaOH solution. Blue colour is obtained.
(c) Tests for Proteins:
(i) Xanthoproteic test. To 5 cc of protein solution, add 2cc of conc. HNO3. Heat with boiling. A yellow colour appears. Cool it and add excess of 20% NH4OH or NaOH. Orange colour is formed. It is due to nitration of phenolic groups attached to side chains of aromatic amino acids. This test is performed for proteins containing aromatic amino acids like tyrosine, tryptophan etc.
(ii) Grind protein rich seeds (Gram, Pea) with water to make protein solution. Add Millons reagent and heat to boiling. Red colour is formed.
(iii) Biuret Test. To 3 ml of protein solution, add 1 ml of 40% NaOH and few drops of 5% CuSO4 solution. Shake and keep for 15 minutes at room temperature. A violet/pink colour appears which gradually changes to blue and purple. Actually Cu++ reacts with CONH of proteins and forms a violet coloured complex called biuret (CONH2 — NH—CONH2).
(iv) Beat white of egg with 8 times its volume of H20. Filter and perform xanthoproteic test.
(d) Test for Amino Acids:
Ninhydrin test is best to detect amino acids. Most of the amino acids give purple colour with ninhydrin but tyrosine, phenylalanine aspartic acid give blue colour; tryptophan produces olive brown and proline gives yellow colour. 0. 2% ninhydrin solution made in 70% alcohol is put on a piece of filter paper and dried.
Now put a drop of aqueous solution of amino acids on this dry filter paper. Dry in oven. A blue/violet/purple colour appears due to the reaction of ninhydrin with amino group of amino acid to form Ruhemann’s purple compound.
(e) Test for Urine:
Urine is tested for presence of urea, uric acid, creatinine, minerals (Cl, SO4, Ca, PO4), sugar and albumin protein.
(i) Urine for presence of urea: Urea when heated decomposes with the liberation of ammonia and the formation of biuret. The biuret is dissolved in water and develops a violet colour forming a complex with alkaline CuSO4 solution.
Place a small amount of urea crystals in a dry test tube and heat it in a low flame. Urea melts and solidifies. (In case of urine, urine is heated to solidify). Cool the test tube. Add 3 ml of water and shake. Add 1 ml of dil NaOH and 2 drops of 1% CuSO4 solution. Pink colour develops indicating presence of urea.
(ii) For detection of sugar in urine perform Benedicts test. To about 5 ml of Benedicts reagent, add 0.5 ml (8 drops) of urine and boil for 2 minutes. A light green/yellow/brick red precipitate indicates the presence of reducing sugar in urine. The intensity of colour depends upon percentage of sugar in urine.
(iii) For albumin in urine, (a) Fill 3/4th of the test tube by urine after filtering it. Heat the upper 1/3rd of the test tube by a small flame. A turbidity is found on the heated portion of the urine. Add a drop of 33% acetic acid to the urine. Phosphate is dissolved but not the albumin protein, (b) Add a few drops of 30% sulphosalicyclic acid to 2 ml of clear filtered urine. A turbidity indicates the presence of albumin.
Note # 17. Feedback Inhibition of Enzymes:
Feedback inhibition (also called end-product inhibition or allosteric modulation) is one in which the end- product of the reaction acts as inhibitor and inhibits the activity of regulatory enzyme, usually, enzyme of the first step of a biosynthetic pathways. In multi-enzyme system synthesis of a product is completed in a number of steps, each step being catalyzed by a specific enzyme.
In some of such systems, the regulatory enzyme is specifically inhibited by the end-product of the pathway whenever the concentration of the end-product exceeds the cell’s requirement. When the reaction catalyzed by the regulatory enzyme is slowed, all subsequent enzymes act at reduced rates due to the depletion of their substrates.
The rate of production of the pathway’s end-product is thereby brought into balance as per the requirement of the cell. Feedback inhibition is beautifully illustrated by biosynthesis of L-isoleucine from L-threonine (Fig. 10-12).
In this system, the first enzyme, threonine dehydratase, is inhibited by L-isoleucine. No other intermediate in the sequence inhibits threonine dehydratase, nor is any other enzyme of the system inhibited by L-isoleucine.
L-isoleucine binds not to the active site, but to regulatory site on the enzyme molecule; this is called allosteric modulation. However, when the concentration of the end-product drops sufficiently, the enzyme reactivates and the end-product is resynthesized.
Note # 18. Specificity of Enzyme:
One characteristic that distinguishes an enzyme from all other types of catalysts is its substrate specificity (Fig. 8.19). Enzyme specificity is a result of the uniqueness of the active site of each enzyme.
Enzyme specificity is arbitrarily grouped as:
i. Absolute specificity:
Enzymes having absolute specificity will catalyse a particular substrate only and will have no catalytic effect on substrates that are closely related. E.g., Urease will catalyse the hydrolysis of urea but not of methyl urea, thiourea or biuret:
ii. Stereo-chemical specificity:
Most enzymes show a markedly high degree of specificity toward one stereo-isomeric form of the substrate, e.g:
(1) Lactic acid dehydrogenase catalyses the oxidation of the L-lactic acid found in muscle cells but not the D-lactic acid found in certain microorganisms.
(2) Fumerase adds water to fumaric acid but not to its cis-isomer-maleic acid.
iii. Group specificity:
Enzymes having group specificity are less selective in that they will act upon structurally similar molecules having the same functional groups, e.g., many of the peptidases.
(1) Pepsin will hydrolyse all peptides having adjacent aromatic amino acids.
(2) Carboxy-peptidase attacks peptides from the carboxyl end of the chain, cleaving the amino acids one at a time.
iv. Linkage specificity:
Enzymes having linkage specificity are the least specific of all, because they will attack a particular kind of chemical bond, irrespective of the structural features in the vicinity of linkage, e.g., lipases catalyse the hydrolysis of ester linkages in lipids.
Note # 19. Factors Influencing Enzyme Activities:
i. Temperature:
An enzyme is active within a narrow range of temperature. The temperature at which an enzyme shows its highest activity is called optimum temperature (Fig. 9.29). It generally corresponds to the body temperature of warm blooded animals, e.g., 37°C in human beings. Enzyme activity decreases above and below this temperature.
Enzyme becomes inactive below minimum temperature and beyond maximum temperature. Low temperature preserves the enzymes in the inactive state. Therefore, it is used in preservation of foods inside cold storages.
Low temperature present inside cold storages prevents spoilage of food by two methods:
(i) Inactivity of enzymes present inside food article and
(ii) Non-activity of microbes because their enzymes also become inactive at low temperature.
High temperature destroys enzymes by causing their denaturation. This occurs at 50°C or so. In between the minimum and maximum temperatures, the reaction velocity doubles for every rise in 10°C (general rule of thumb). A time factor appears beyond optimum temperature. Here there is a rise in velocity for a short time followed by a sharp fall.
As opposed to warm blooded or homoeothermal animals (mammals, birds), there are cold blooded or poikilothermal animals (reptiles, amphibians, fishes, invertebrates) whose body temperature rises or falls with that of environmental temperature.
These animals cannot live in very hot or very cold environment as enzyme functioning will be impaired. Because of this reason, frog seeks moist shady environment during summer and lies in an inactive form (hibernation) in the deeper layers of the soil during winter.
ii. Optimum pH:
Every enzyme has an optimum pH when it is most effective. A rise or fall in pH reduces enzyme activity by changing the degree of ionisation of its side chains.
A change in pH may also start reverse reaction. Fumarase catalyses fumarate → malate at 6.2 pH and reverse at 7.5 pH. Most of the intracellular enzymes function near neutral pH with the exception of several digestive enzymes which work either in acidic range of pH or alkaline, e.g., 2.0 pH for pepsin, 8.5 for trypsin.
iii. Enzyme Concentration:
The rate of a biochemical reaction rises with the increase in enzyme concentration up to a point called limiting or saturation point (Fig. 9.30). Beyond this, increase in enzyme concentration has little effect.
iv. Product Concentration:
If the products are allowed to remain in the area of the reaction, the rate of forward reaction will fall. Reverse reaction can also start.
v. Activators:
They increase activity of enzymes (e.g., chloride for salivary amylase), function as cofactors (e.g., K+, Mn2+) and convert pro-enzymes to enzyme state. HCl of digestive juice changes pro-enzyme pepsinogen to enzyme pepsin. Pepsin also possesses autocatalytic property as it can also change pepsinogen to pepsin state.
vi. Protein Poisons:
Cyanides, azides, iodoacetate, and salts of heavy metals destroy tertiary structure of enzymes by either combining with cofactor or a group of apoenzyme (—SH group, —COOH).
vii. Radiation Energy:
High energy radiations break hydrogen bonds, ionic bonds, and other weak linkages to destroy enzyme structure.
viii. Substrate Concentration:
Increase in substrate concentration increases the rate of reaction.
The enhanced rate is due to two factors:
(a) Occupation of more and more active sites by the substrate molecules;
(b) Higher number of collisions between substrate molecules.
The rise in velocity is quite high in the beginning but it decreases progressively with the increase in substrate concentration. If a graph is plotted for substrate concentration versus reaction velocity, it appears as a hyperbolic curve.
A stage is reached where velocity is maximum. It does not increase further by increasing the substrate concentration. At this stage the enzyme molecules become fully saturated and no active site is left free to bind additional substrate molecules. This saturation effect is shown by all enzymes. Because of this Victor Henri (1903) proposed the formation of enzyme-substrate complex as an essential step in enzyme catalysis.
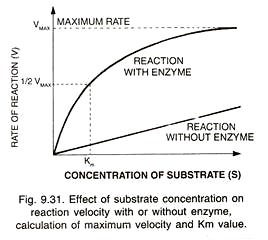
Michaelis Constant (Michaelis Menten Constant, Km). It is a mathematical derivation or constant which indicates the substrate concentration at which the chemical reaction catalysed by an enzyme attains half its maximum velocity (Fig. 9.31).
Constant was given forth by Leoner Michaelis and Mand Menten (1913). Km or Michaelis Menten constant generally lies between 10- 1 to 10-6 M. Km indicates affinity of the enzyme for its substrate.
A high Km indicates low affinity while a low Km shows strong affinity. If an enzyme acts on more than one substrate it shows different Km values for them. Thus enzyme protease acts on large number of proteins. Its Km value will differ from protein to protein.
Allosteric enzymes do not show a typical Michaelis Menten constant or behaviour. The classical hyperbolic curve is replaced by a sigmoid saturation curve.
Note # 20. Biochemical Pathways of Enzymes:
All chemical reactions occurring in the living systems are mediated through organic catalysts called enzymes. Like catalysts, enzymes remain unchanged at the end of the chemical reactions. They can, therefore, be used and reused. Catalysts (e.g., Platinum) are relatively unselective but enzymes are highly specific.
There is a separate enzyme for every biochemical reaction, i.e., there is no un-catalysed metabolic conversion in living systems. Even an otherwise physical process like dissolution of carbon dioxide in water is catalysed in living systems.
It is because rate of enzyme catalysed reaction is hundreds of times higher than the rate of un-catalysed reaction. In the absence of enzyme, nearly 200 molecules of carbon dioxide dissolve in water per hour to form carbonic acid. In the presence of enzyme carbonic anhydrase, some 600,000 molecules of carbonic acid are formed per second. This is an acceleration of about 10 million times.
During chemical reactions, older chemical bonds are broken and new chemical bonds are established, e.g.,
It is an inorganic chemical reaction. Organic chemical reactions also occur similarly. Rate of chemical or a physical reaction is determined by the amount of product formed per unit time.
Rate = δP/δt
Rate of chemical reaction doubles or decreases by half for every 10°C rice or fall. It is called general rule of thumb. As there are thousands of chemical reactions occurring in living cells, thousand of different types of enzymes develop inside the cells. Enzymes are generally globular proteins having one or more clefts over their surface. The clefts function as active sites. Active sites attract substrate molecules or reactants.
An enzyme-substrate complex is formed. Chemical reaction occurs in this stage. It forms products. The products leave the active site which becomes free to attract more substrate molecules. The active site of each type of enzyme is specific for its substrate. This explains the specificity of enzymes. Sucrase will act only on sucrose and no other disaccharide.
Types:
Metabolism is of two kinds, catabolism and anabolism. Anabolism includes all the “building up” reactions.
It is also called constructive metabolism since it involves the synthesis of complex substances from simpler ones, e.g., synthesis of organic compounds from CO2 and H2O during photosynthesis, formation of starch from glucose, production of proteins from amino acids, formation of lipids from fatty acids and alcohols. Energy is stored (as potential energy) in the process.
Catabolism (= katabolism) constitutes “breakdown reactions”. It is also known as destructive metabolism because it involves breaking of complex substances into simpler ones. Potential energy present in the complex substances is converted into kinetic energy.
Same of energy is trapped as chemical energy in adenosine triphosphate (ATP). The latter is also called energy currency of living systems. Respiration is an example of catabolism. It releases energy for performing different body activities.
Metabolism, therefore, involves changes in energy and materials. Bioenergetics is the scientific study of energy transformations in the living systems, e.g., organisms, ecosystems. Biochemical Pathways are Tightly Regulated. Biochemical reactions do not occur singly.
Neither they are unregulated. Unregulated anabolic and catabolic reactions occurring simultaneously can create chemical chaos as a substance synthesized in anabolism will be immediately broken down in catabolism. There can be excess synthesis or breakdown of a material resulting in unnecessary wastage of energy and materials.
This does not happen. Actually cells have well separated biochemical pathways which are under tight control of regulatory systems which include control of enzyme synthesis, activation and inhibition of enzymes and feedback systems.
Most enzymes have allosteric sites away from active sites. Presence of an activator over the allosteric site makes the active site functional while occurrence of an inhibitor over the allosteric site dysfunctions the active site.
Further, a metabolic or biochemical pathway has a number of steps, each controlled by a separate enzyme. The pathway becomes operational only when the substrate of the first reaction and its active enzyme are available. The product of the first reaction generally becomes the substrate of the second reaction catalysed by a separate enzyme.
The product of the second reaction becomes the substrate of the third reaction and so on till the final product is formed. The cells control the amount of final product as per their requirement by either controlling availability of the first substrate, first enzyme, utilisation of intermediate product or feedback mechanism.
In feedback mechanism or inhibition, the excess final product functions as allosteric inhibitor of the first enzyme, e.g., glucose 6-phosphate for enzyme hexokinase.
The Living State:
Hundred and thousands of metabolites or biomolecules occur in organisms in concentration characteristics of each of them, e.g., glucose is 4.5-5.0 mM in blood, hormones in Nano gram/ml.
The living systems maintain this concentration of biomolecules because they are in metabolic flux, always remaining in non-equilibrium steady state where equilibrium is seldom achieved. No work can be carried out in equilibrium state.
Therefore, living systems are regularly receiving an input of energy to prevent reaching an equilibrium and remain always in non-equilibrium steady state. Energy is obtained from metabolism. Metabolism and living state are, therefore, complementary and synonymous. There cannot be a living state without metabolism.
Note # 21. Regulation of Enzyme:
Biochemical reaction studies have shown that the pace of a chemical reaction in a biological system is maintained by the activities of the enzymes. Enzymes are rather unstable molecules and are synthesized and degraded simultaneously. Their activities may be regulated either through their synthesis or by modifying the existing enzyme molecules.
The activities of enzyme molecules are regulated by several ways which are the following:
I. Allosteric Regulation:
Allosteric regulation is a fine mechanism of controlling a reaction through the enzyme activity. Some enzymes (called allosteric enzymes), show sigmoidal curve between the substrate concentration and the activity. The activity of these enzymes is modified by several metabolites. The effect of different concentrations of ‘activator’ and ‘inhibitor’ on these enzymes is also sigmoid.
These effector molecules have a structure different from the substrate molecules. In most of the cases, allosteric inhibitors are the end products of the reaction; inhibiting the first enzyme in the series.
Thus, this kind of inhibition is called feedback inhibition, end product inhibition or retro-inhibition. The allosteric activators arc normally one of the substrates or cofactors of the enzyme. The effect of the allosteric ‘inhibitor’ or ‘activator’ on the enzyme is reversible.
When they are withdrawn, the enzyme resumes the original activity:
i. Allosteric Inhibition:
Inhibition of threonine deaminase by isoleucine is an example of allosteric inhibition. Threonine deaminase deaminates threonine to α-ketrobutyrate. The final product of the reaction is isoleucine.
Whenever the accumulation of isoleucine occurs, conversion of threonine to α-ketobutyrate and consequently formation of other intermediaries in the biosynthesis of isoleucine is stopped. When isolcucine is used up, threonine deaminase is reactivated and reactions for the biosynthesis of isoleucine start again.
ii. Allosteric Activation:
Activation of glycogen synthetase by glucose-6-phosphate is an example of allosteric activation. Another example of allosteric regulation (of both inhibitory and activating type) is observed during Pasteur effect. Pasteur effect is the inhibition of glycolysis and fermentation by oxygen. The molecular basis of this effect is the allosteric inhibition of enzyme phosphofructokinase by ATP and citrate and its activation by AMP.
Like many others, this kind of regulation is also of adaptive significance. As the level of AMP increases due to increased use of ATP in the cell, glycolysis is increased by the activation of phosphofructokinase with the result of more formation of ATP. When ATP level exceeds normal requirement of the cell, inhibition of glycolysis occurs through the same enzyme phosphofructokinase and ATP synthesis is stopped.
iii. Mechanism of Allosteric Regulation:
Regarding the mechanism of allosteric regulation, it is proposed that allosteric enzymes have two active centers; one for the substrate and the other for effector. These two sites lie either on same or on two different subunits. Binding of a effector molecule to one type of subunit changes the structure of the enzyme molecule in such a way that binding of the substrate to the other subunit is affected.
To explain the mechanism, an example of allosteric regulation of aspartate transcarbamylase may be cited. Asparate transcarbamylase contains two types of subunits. These two types of subuntis may be split apart by treatment with mercurials; with one type retaining the ability to bind with the substrate, whereas the other to recognize the inhibitor.
When these two species of subunits are together (active enzyme molecule), binding of the inhibitor to one type of subunit changes the structure of other subunits in such a way that the binding of the substrate is inhibited. When the subunits containing binding sites for the inhibitor are removed enzyme is not affected by the inhibitor.
Further, it gives a typical Michaelis-Menten curve with the substrate concentration. Similarly, the binding of activator may change the molecular structure in such a way that the binding of substrate is facilitated.
II. Isozyme Formation:
Another phenomenon that controls cellular metabolism is the formation of isozymes (isoenzymes). Isozymes are different physical forms of the same enzyme performing the same general function at different rates. They differ to some extent in their amino acid composition also, so that they may be separated by electrophoresis. Lactate dehydrogenase is a classic example of isozyme formation. It catalyses the oxidation of lactate to pyruvate with the help of NAD+.
Lactate dehydrogenase enzyme is a tetramer composed of two distinct types (H and M types) of subunits.
Depending upon the relative number of two types of subunits, lactate dehydrogenase forms 5 isozymes as follows:
LD1 = HHHH
LD2 = HHHM
LD3 = HHMM
LD4=HMMM
LD5 = MMMM
The molecular weight of the enzyme is 13,500 but when it is treated with urea or guanidine hydrochloride, it dissociates into subunits each having a molecular weight of about 35,000. The regulation of different isozymes is different. LD1 (HHHH) type of lactate dehydrogenase is found in the heart muscles.
This species is most active at low pyruvate concentration and is inhibited by high concentrations of pyruvate. LD5 (MMMM) type of enzyme is found in skeletal muscle cells and it remains active at high pyruvate concentrations.
Another example of isozyme formation is that of aspartokinase. This enzyme catalyzes the reaction between aspartic acid and ATP to form aspartyl phosphate. Amino acids lysine, methionine, and threonine are final products of the reaction.
The enzyme aspartokinase exists in three forms—aspartokinase I, aspartokinase II and aspartokinase III. Aspartokinase I is inhibited by threonine and III by lysine. Aspartokinase II is insensitive to any of these amino acids. Thus, when any one of these amino acids accumulates, the synthesis of the other is affected very little.
III. Multienzyme System:
Some enzymes exist not as individuals but as aggregates of several enzymes and coenzymes. This they do to channel the metabolities in a pathway efficiently. In an aggregate, each component is arranged in a way that the product of one enzyme becomes the substrate for the other and so on.
An example of enzyme aggregation is that of pyruvic acid dehydrogenase of E. coli. This complex consists of three enzymes- pyruvate decarboxylase, dihydrolipoic dehydrogenase and lipoyl reductase transacetylase. The coenzyme associated with the complex are thiamine pyrophosphate (TPP) and flavin adenine dinucleotide (FAD). A schematic diagram of pyruvate dehydrogenase complex is given in Fig. 27.13.
The stepwise reactions catalyzed by this complex may be written as follows:
Pyruvate + thiamine pyrophosphate → α-hydroxythyl thiamine + pyrophosphate + CO2
α -hydroxyethyl thiamine + lipoate → Thiamine pyrophosphate + acetyl dihydrolipoate
Acetyl dihydrolipoate + CoASH → Acetyl CoA + dihydrolipoate
Dihydrolipoate + NAD+/FAD+ → Lipoate + NADH/FADH2
IV. Regulation by Adenylate Energy Charge:
The importance of adenosine phosphates in metabolic processes has been well recognised for living systems. The adenylate energy charge is the measure of total pool of adenosine phosphates in the form of ATP, ADP and AMP. D.D. Atkinson (1969) defines adenylate energy charge as follows.
In most systems, an increase in adenylate energy charge in the physiological range results in stimulation of regulatory enzymes. Although it is a well-known phenomenon in animals and microorganisms, some instances have been recorded from plants also.
It has been shown that the adenylate energy charge affects the activity of pyrophosphomevalonate decarboxylase, which is the key enzyme in the biosynthesis of kaurene from mevalonate. An increase in enzyme activity is observed between adenylate energy charge of 0.8 and 1.0.