ADVERTISEMENTS:
Let us make in-depth study of the two types of nitrogen fixation.
The two types of nitrogen fixation are: (1) Physical Nitrogen Fixation and (2) Biological Nitrogen Fixation.
Apart from carbon, hydrogen and oxygen, nitrogen is the most prevalent essential macro-element in living organisms. Plants need nitrogen to build amino acids, proteins, nucleic acids, cytochromes, chlorophylls, alkaloids, phytohormones and many of the vitamins. Plants compete with microbes for limited nitrogen content available in the soil. Plants mainly absorb nitrogen in the form of nitrate (NO3–) or ammonium ions (NH4+) from the soil.
ADVERTISEMENTS:
The nitrate is more abundant in well oxygenated, non-acidic soils, while ammonium is predominant in acidic or water logged soils. The other sources of available soil nitrogen may be amino acids from decaying organic matter, animal excreta (urea) and chemical fertilizers that can be absorbed directly by the plants. Nitrogen is obtained by the plants mainly from the atmosphere. It occurs as free diatomic (N2) molecules in the air. It is highly inert gas. It cannot be used directly by the higher plants, and therefore has to be fixed.
The phenomenon of conversion of free nitrogen (molecular and elemental) into nitrogenous compounds (to make it available to the plants for absorption) is called nitrogen fixation. Nitrogen fixation is carried out by physicochemical and biological means. About 10% of natural nitrogen fixation takes place by physicochemical methods and 90% by biological methods.
These are briefly discussed below:
(1) Physical Nitrogen Fixation:
(i) Natural Nitrogen Fixation:
ADVERTISEMENTS:
Under the influence of lightning (i.e., electric discharge in the clouds) and thunder, N2 and O2 of the air react to form nitric oxide (NO). The nitric oxides are again oxidized with oxygen to form nitrogen peroxide (NO2).
The reactions are as follows:
N2 + O2 Lightning → Thunder 2N0 (Nitric Oxide); 2NO + O2 → 2NO2 Oxidation (Nitrogen peroxide)
During the rains, NO2 combines with rain water to form nitrous acid (HNO2) and nitric acid (HNO3). The acids fall on the soil along with rain water and react with the alkaline radicals to form water soluble nitrates (NO3-) and nitrites (N02-).
2NO2 + H2O → HNO2 + HNO3; HNO3 + Ca or K salts → Ca or K nitrates
The nitrates are soluble in water and are directly absorbed by the roots of the plants.
(ii) Industrial Nitrogen Fixation:
Ammonia is produced industrially by direct combination of nitrogen with hydrogen (obtained from water) at high temperature and pressure. Later, it is converted into various kinds of fertilizers, such as urea etc.
2. Biological Nitrogen Fixation:
The conversion of atmospheric nitrogen into the nitrogenous compounds through the agency of living organisms is called biological nitrogen fixation. The process is carried out by two main types of microorganism: those which live in close symbiotic association with other plants and those which are “free living” or non-symbiotic.
ADVERTISEMENTS:
Biological nitrogen fixation (BNF) is the process whereby atmospheric nitrogen is reduced to ammonia in the presence of nitrogenize. Nitrogenize is a biological catalyst found naturally only in certain microorganisms such as the symbiotic Rhizobium and Frankia, or the free-living Azospirillum and Azotobacter and BGA.
Details of biological nitrogen fixation follow.
Nearly 80% of Earths atmosphere contains nitrogen in the form of a highly inert di-nitrogen (N = N) which most plants cannot utilize as such. The atmospheric di-nitrogen (N2) consists of two nitrogen atoms linked by a triple-covalent bond. About 225 kcal of energy is required to break this triple bond which is difficult to achieve.
The phenomenon of reduction of inert gaseous di-nitrogen (N2) into ammonia (NH3) through the agency of some microorganisms so that it can be made available to the plants is called as biological nitrogen fixation or diazotrophy.
ADVERTISEMENTS:
Nitrogen Fixers:
Among the earth’s organisms, only some prokaryotes like bacteria and cyanobacteria can fix atmosphere nitrogen. They are called nitrogen fixers or diazotrophs. They fix about 95% of the total global nitrogen fixed annually (-200 million matric tones) by natural process.
Diazotrophs may be asymbiotic (free living) or symbiotic such as given below:
(i) Free Living Nitrogen Fixing Bacteria:
ADVERTISEMENTS:
Azotobacter, Beijerinckia (bothaerobic) and Clostridium (anaerobic) are saprophytic bacteria that perform nitrogen fixation. Desulphovibrio is chemotrophic nitrogen fixing bacterium. Rhodopseudomonas, Rhodospirillum and Chromatium are nitrogen fixing photoautotrophic bacteria. These bacteria add up to 10-25 kg, of nitrogen/ha/annum.
(ii) Free living Nitrogen Fixing Cyanobacteria:
Many free living blue-green algae (now called cyanobacteria) perform nitrogen fixation, e.g., Anabaena, Nustoc, Aulosira, Cylmdrospermum, Trichodesmium. These are also important ecologically as they live in water-logged sods where denitrifing bacteria can be active. Aulosira fertilissima is the most active nitrogen fixer in Rice fields, while Cylindrospermum is active in sugarcane and maize fields. They add 20-30 kg Nitrogen/ha/annum.
(iii) Symbiotic Nitrogen Fixing Cyanobacteria:
ADVERTISEMENTS:
Anabaena and Nostoc species are common symbionts in lichens, Anthoceros, Azolla and cycad roots. Azolla pinnata (a water fern) has Anabaena azollae in its fronds. It is often inoculated to Rice fields for nitrogen fixation.
(iv) Symbiotic Nitrogen Fixing Bacteria:
Rhizobium is aerobic, gram negative nitrogen fixing bacterial symbionts of Papilionaceous roots. Sesbania rostrata has Rhizobium in root nodules and Aerorhizobium in stem nodules. Frankia is symbiont in root nodules of many non-leguminous plants like Casuarina and Alnus.
Xanthomonas and Mycobacterium occur as symbiont in the leaves of some members of the families Rubiaceae and Myrsinaceae (e.g., Ardisia). Several species of Rhizobium live in the soil but are unable to fix nitrogen by themselves. They do so only as symbionts in the association of roots of legumes.
Symbiotic Nitrogen Fixation:
Both Rhizobium sp. and Frankia are free living in soil, but only as symbionts, can fix atmospheric di-nitrogen.
ADVERTISEMENTS:
The symbiotic nitrogen fixation can be discussed under following steps:
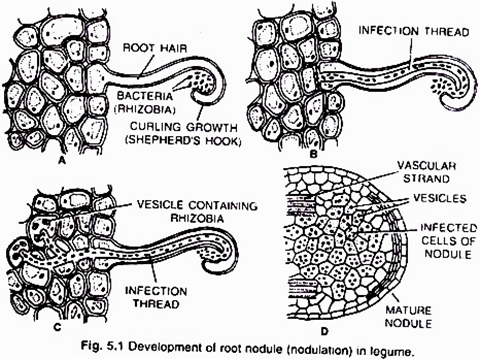
(i) Nodule formation (Fig. 5.1):
It involves multiple interactions between free-living soil Rizobium and roots of the host plant. The important stages involved in nodule formation are as follows-Host Specificity: A variety of microorganisms exist in the rhizosphere (i.e. immediate vicinity of roots) of host roots.
The roots of young leguminous plants secrete a group of chemical attractants like flavonoids and betaines. In response to these chemical attractants specific rhizobial Tells migrate towards the root hairs and produce nod (nodulation) factors. The nod factors found on bacterial surface bind to the lectin proteins present on the surface of root hairs. This lectinnod factor interaction induces growth and curling of root hairs around Rhizobia.
At these regions wall degrades in response to node-factors and Rhizobia enter the root hair invagination of plasma membrane called infection thread. The infection thread filled with dividing Rhizobia elongate through the root hair and later branched to reach different cortical cells.
ADVERTISEMENTS:
The Rhizobia are released into the cortical cells either single or in groups enclosed by a membrane. The Rhizobia stop dividing, loose cell wall and become nitrogen fixing cells as led bacteroids .The membrane surrounding the bacteroids is called peribacteroid membrane. The infected cortical cells divide to form nodule (Fig. 5.2).
(ii) Mechanism of nitrogen fixation (Fig 5.3):
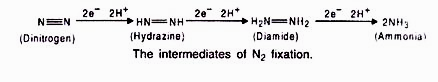
The nodule serves as site for N2 fixation. It contains all the necessary bio-chemicals such as the enzyme complex called nitrogenase and leghaemoglobin (leguminous haemoglobin). The nitrogenase has 2 components i.e. Mo-Fe protein (molybdoferredoxin) and Fe-protein (azoferredoxin).The nitrogenase catalyzes the conversion of atmosphere di-nitrogen (N2) to 2NH3. The ammonia is the first stable product of nitrogen fixation.
The overall equation is:
The nitrogenase is extremely sensitive to oxygen. To protect these enzymes, nodule contains an oxygen scavenger called leghaemoglobin (Lb), which is a reddish-pink pigment. There are two views about location of leghaemoglobin that is either located outside the peribacteroid membrane or located in between bacteroids.
During nitrogen fixation, the free di-nitrogen first bound to MoFe protein and is not released until completely reduced to ammonia. The reduction of di-nitrogen is a stepwise reaction in which many intermediates are formed to form ammonia (NH3) which is protonated at physiological pH to form NH4+. In this process ferredoxin serves as an electron donor to Fe-protein (nitrogenase reductase) which in turn hydrolyzes ATP and reduce MoFe protein, the MoFe protein in Turn reduce the substrate N2. The electrons and ATP are provided by photosynthesis and respiration of the host cells.
Assimilation of Ammonia:
The ammonia produced by nitrogenase is immediately protonated to form ammonium ion (NH4+). As NH4+ is toxic to plants, it is rapidly used near the site of generation to synthesize amino acids. Amino acids synthesis takes place by three methods: reductive animation, catalytic amination and transamination.
(i) Reductive amination:
In this process, glumate dehydrogenase (GDH) catalyzes the synthesis of glutamic acid.
(ii) Catalytic amidation:
It is a two step process catalyzed by glutamine synthetase (GS) and glutamate synthetase (glutamine – 2-oxyglutarate aminotransferase, or GOGAT).
Out of the two glutamates produced one returns to GS while the other is exported to the plant.
(iii) Transamination:
Glutamate or glutamic acid is the main amino acid from which other amino acids are derived through transamination. The enzyme aminotransferases (= transaminases) catalyze all such reactions. Transamination involves transfer of amino group from one amino acid to the keto group of keto acid.
Glutamate (amino donor) + Oxaloacetate (amino acceptor) → Aspartate (amino acid) + 2 oxyglutarate
In nitrogen fixing plants, the fixed nitrogen is exported in the form of amides (asparagines and glutamine) and Ureides (allantoin, allantoic acid and citrulline), from the nodules to other plant parts via xylem. Amides are formed from two amino acids, namely glutamic acid and aspartic acid, by replacing – OH part by another NH2– radicle. Thus, amides contain more nitrogen than amino acids and are structural part of most proteins.
Nitrate Assimilation:
Nitrate cannot be utilized by plants as such. It is first reduced to ammonia before being incorporated into organic compounds. Reduction of nitrate occurs in two steps:
1. Reduction of nitrate to nitrite:
It is carried out by an inducible enzyme, nitrate reductase. The enzyme is a molybdoflavoprotein. It requires a reduced coenzyme NADH or NADPH for its activity which is brought in contact with nitrate by FAD or FMN.
2. Reduction of nitrate:
It is carried out by the enzyme nitrite reductase. The enzyme is a metalloflavoprotein which contains copper and iron. It occurs inside chloroplast in leaf cells and leucoplast of other cells. Nitrite reductase require reducing power. It is NADPH and NADH (NADPH in illuminated cells).
Reduction process also require ferredoxin which occurs in green tissues of higher plants. It is presumed that in higher plants either nitrite is trans-located to leaf cells or some other electron donor (like FAD) operates in un-illuminated cells. The product of nitrite reduction in ammonia.
Ammonia thus produced combines with organic acids to produce amino acids. Amino acids form protein by the process of translation.