ADVERTISEMENTS:
Read this article to learn about the nitrogen cycle and the oxygen cycle.
The Nitrogen Cycle:
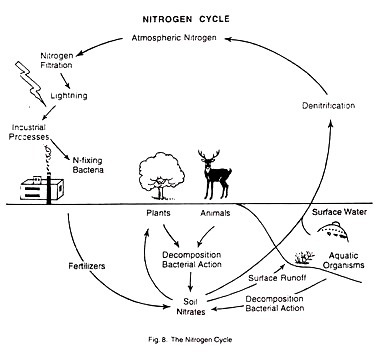
The most abundant element in the atmosphere is nitrogen. In its elemental form it is a colourless and odorless gas which cannot be used by plants or animals. But in combination with oxygen or other elements, nitrogen is available to living organisms as a nutrient. The nitrogen cycle may be defined as the circular flow of nitrogen from free nitrogen gas in the atmosphere to nitrates in the soil, and, finally, back to atmospheric nitrogen.
Nitrogen Fixation:
ADVERTISEMENTS:
By this process, nitrogen gas is converted to nitrates.
This can be done in many ways:
(i) There are some Nitrogen-fixing bacteria that live in the soil or in nodules on the roots of leguminous plants. They can convert nitrogen gas to nitrate. Nitrogen fixation is also done by some types of blue-green algae and fungi.
(ii) Nitrogen fixation also occurs in the atmosphere. This is natural nitrogen fixation. It takes place when lightning occurs, because the electrical current that passes through the atmospheric nitrogen converts some of the nitrogen gas to nitrogen compounds. These compounds can be used by plants.
ADVERTISEMENTS:
(iii) Nitrates are also released from dead and decaying plants and animals and animal wastes.
De-nitrification:
At the same time, when nitrates are being produced from nitrogen gas, other nitrates are breaking down and releasing nitrogen gas back to the atmosphere. This process of reversal is called de-nitrification.
De-nitrification Occurs:
(i) When some forms of bacteria come in contact with nitrates, and also,
(ii) When run-off water carry nitrates into surface water which constantly exchanges nitrogen with the atmosphere.
There are some ecosystems where the process of nitrogen fixation and de-nitrification are attuned according to the productivity demands of the ecosystems. The processes are the most rapid in the temperate ecosystems, especially during winter.
The Oxygen Cycle:
The element oxygen can be said to be ubiquitous. It is chemically extremely reactive and can combine with a wide range of other elements. For instance, oxygen combines with hydrogen to form water (H2O); in combination with carbon forms carbon monoxide (CO) and carbon dioxide (CO2); with sulphur it forms sulphur dioxide (SO2), and similar other compounds with other elements. Oxygen is an important constituent of organic substances like sugars, cellulose and starches. It is found in almost all minerals and rocks. The biogeochemical cycling of oxygen is very complex.
ADVERTISEMENTS:
Two reasons are mainly responsible for this:
(i) Oxygen has a high degree of chemical reactivity, and
(ii) Oxygen is present in a wide range of biotic and abiotic ecosystem components.
Oxygen in its elemental state is known as free oxygen.
ADVERTISEMENTS:
There are two main pools of free oxygen:
(i) The atmosphere contains about 0.8 x 1015t (Johnson 1970); and
(ii) The oceans contain about 0.2 X 1015t.
The production and renewal of these two pools are carried out by the two following processes:
ADVERTISEMENTS:
(i) Photo dissociation of water vapour in the upper atmosphere, as a result of which oxygen is released when water molecules are split by light;
(ii) Photosynthesis, by which energy is fixed and oxygen is released. Photosynthesis is the major contributor of molecular oxygen.
The ecosystems of the past produced more oxygen than was used in respiration, oxidation of rocks or the decay of organic matter. The presence of fossil fuels represents reduced carbon withdrawn from the bio-geochemical cycles. They prove the lack of oxidation and the decay of some types of ancient ecosystems.
It is usually difficult to assess global biogeochemical cycles. But it is said that the oxygen cycle is a perfect cycle in a state of dynamic equilibrium, in spite of anthropogenic activity that involves fossil fuel burning. The burning of fossil fuel releases annually 2,000 times greater carbon than is stored. As a result, oxygen is rapidly removed from the atmosphere.
ADVERTISEMENTS:
In 1970, Machta and Hughes said that between 1910 and 1970, fossil fuel burning has not had any discernible effect on oxygen concentrations. The cycle has maintained a steady state condition probably because it has negative feedback mechanisms.
According to Redfield (1958), sulphate-reducing bacteria, which are found in anaerobic environment, may operate in such a way. These chemosynthetic bacteria are capable of using sulphate ion as a source of oxygen.
This can be shown as:
In this process, free oxygen is not released, but organic matter is decomposed and nutrients are released. The nutrients are phosphates, nitrates and carbon dioxide. Subsequently, carbon dioxide may be fluxed to a situation where it is used in photosynthesis and consequently oxygen is released.
However, it should be kept in mind that these bacteria survive only in anerobic habitats. Therefore, any change in the content of atmospheric oxygen would reflect the abundance of anerobic habitats. As a result, bacteria populations will increase and this would regulate oxygen supply.
An increased concentration of carbon dioxide in the atmosphere may increase photosynthesis and production of molecular oxygen. At the same time, pollution, rampant deforestation and other anthropogenic activities may reduce photosynthesis. This will lead to an imbalance in both the carbon and oxygen cycles.