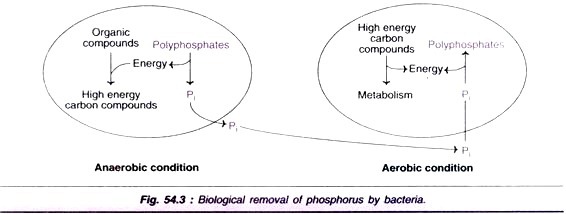
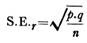ADVERTISEMENTS:
The following points highlight the top three staining methods used for colouring the biological materials. The types are: 1. Negative Staining 2. Simple Staining 3. Differential Staining.
Method # 1. Negative Staining:
For quicker observation of the morphology of bacterial or yeast cells, the negative staining technique is applied.
Requirements:
ADVERTISEMENTS:
1. Bacterial/yeast suspension.
2. Slide, inoculating needle, pipettes etc.
3. Burner.
4. Glass marker.
ADVERTISEMENTS:
5. Nigrosin dye solution (1%aq.)
6. Microscope.
Procedure:
A drop of bacterial suspension is placed on a clean grease free slide. Then about 1 ml. suspension is spread by a needle on the slide and dried in air. Finally the dry smear is heat fixed by passing through a flame. Then a few drops of nigrosin dye solution is poured on the smear and spread uniformly on the slide. Finally the smear with dye is dried and examined microscopically.
Observation:
Bacteria or yeast cells are seen as glossy bodies against a dark background.
Method # 2. Simple Staining:
I. By Ziehl’s Carbol Fuchsin:
ADVERTISEMENTS:
For facilitating observation under microscope, it is necessary to stain bacteria with suitable dyes. Usually basic dyes are employed for staining bacteria.
Requirements:
(a) Staining reagent –
Basic fuchsin – 1 gm.
ADVERTISEMENTS:
Absolute alcohol – 10 ml.
Phenol (5% aq.) – 100 ml.
(b) Slide, needle, burner, pipette, microscope etc.
Procedure:
ADVERTISEMENTS:
Transfer a small drop of a bacterial suspension to the centre of a grease free slide and spread uniformly over an area of about 1×2 cm. Allow the smear to dry in air and then fix it by passing 3 to 4 times rapidly over a flame.
Place the slide on the edge of a petridish and cover the smear with carbol fuchsin stain. Allow the stain to react for 2 minutes and then wash the slide with running tap water. Dry the slide (Fig. 4.2a.). Observe under microscope using oil immersion objective.
Observation:
ADVERTISEMENTS:
Bacterial cells look purplish.
II. By Crystal Violet:
Requirements:
1. Crystal violet dye solution (0.5% aq.)
2. Slide, needle, burner, pipette, microscope etc.
Procedure:
ADVERTISEMENTS:
1. Take 1 or 2 loopful of a bacterial suspension on a clean slide. Make a thin smear on the slide and allow it to dry. Fix the film by passing the dried slide 3 or 4 times rapidly over a flame.
2. Cover the slide with the stain and allow it to act for 1 minute.
3. Pour off stain, wash with water and dry between blotting papers.
4. Examine the slide under a microscope using both low and high power objectives.
Observation:
Note the shape of the organisms as observed. Bacterial cells take violet coloration (Fig. 4.2b).
Method # 3. Differential Staining:
I. Gram Staining:
This method is used for classification of microorganisms according to their staining property. Certain bacteria when treated with a dye like crystal (or methyl) violet and then with iodine, the stain becomes “fixed” so that subsequent treatment with a decolorizing agent e.g., alcohol or acetone – does not remove the colour. Some organisms, however, are decolorized by this process.
Species retaining the colour are termed “Gram positive” and those decolorized are termed “Gram negative”. In order to render the decolorized organisms visible and to distinguish them from those retaining the colour, a contrast or counterstain is then applied, generally the first stain is violet and can be differentiated from counterstain which is red.
Observation:
Requirements:
1. First stain: (Crystal violet or Methyl violet) – 5 gm.
Distilled Water – 1 lit.
2. Iodine Solution
Iodine – 10 gm.
Potassium Iodide – 20 gm.
Distilled Water – 1 lit.
Dissolve 20 gm. KI in 250 ml. water and then add 10 gm. I2 when dissolved, make up to 1 lit.
3. Counterstain
Safranin – 5 gm.
Distilled Water – 1 lit.
4. Absolute alcohol or 80% acetone.
5. Pipettes, dropper, microscope, blotting paper etc.
Procedure:
1. Make smear (as instructed in earlier expt.), dry, and then heat fix it.
2. Cover the slide with crystal (or methyl) violet solution and allow to act for about 30 – 60s.
3. Pour off stain and holding the slide at an angle downwards pour the iodine solution to wash away the crystal violet. Cover the slide with fresh iodine solution and allow it to act for about 30 – 60s.
4. Wash of the iodine solution with absolute alcohol until colour ceases to come out of the preparation.
5. Wash with water. Rinse with a small amount of counterstain.
6. Apply the counterstain for 1 – 2 minutes.
7. Wash with water and dry between blotting papers.
8. Examine under microscope with oil immersion objective.
Explanation of Gram Staining:
The most plausible explanation for this phenomenon is associated with the structure and composition of bacterial cell wall. Differences in the thickness of cell walls between these two groups of bacteria may be important; the cell walls of gram negative bacteria are generally thinner than those of gram positive bacteria.
Gram-negative bacteria contain a higher percentage of lipid than do gram positive bacteria. Experimental evidence suggests that during staining alcohol treatment extracts the lipid, which results in increased porosity or permeability of the cell wall. Thus the crystal violet-iodine (CV – I) complex can be extracted and the Gram- negative organism is decolorized.
These cells subsequently take on the colour of safranin counter stain. The cell walls of Gram-positive bacteria, because of their different composition (lower lipid content), become dehydrated during treatment with alcohol. The pore size decreases, permeability is reduced, and the CV -I complex cannot be extracted. Therefore, these cells remain purple-violet.
Another explanation, somewhat similar, is also based on permeability differences between the two groups of bacteria. In Gram-positive bacteria, the CV – I complex is trapped in the wall following ethanol treatment, which presumably causes a diminution in the diameter of the pores in the cell- wall peptidoglycan.
Walls of Gram-negative bacteria have a very much smaller amount of peptidoglycan, which is less extensively cross-linked than that in the walls of Gram-positive bacteria. The pores in the peptidoglycan of Gram-negative bacteria remain sufficiently large even after ethanol treatment to allow the CV – I complex to be extracted.
Although Gram-negative organisms consistently fail to retain the primary crystal violet stain, Gram-positive organisms may sometimes show variations in this respect, i.e., a Gram variable reaction. For example, old cultures of Gram- positive bacteria lose the ability to retain crystal violet and hence will be stained by safranin.
II. Endospore Staining:
Mature endospores of bacteria are not easily stained due to the presence of an impervious layer between the spore cytoplasm and the spore coat.
Special, concentrated stains do, however, penetrate. One such stain is Malachite green.
Staining Method: I
Requirements:
(i) Saturated solution of Malachite Green (Malachite green dissolved in dist. water).
(ii) Safranin 0.25% aq. solution.
(iii) 72 hour old bacterial culture suspension.
Procedure:
Prepare the bacterial smear in the usual way and fix by passing the slide 20 times over a flame. Cover the smear with malachite green and allow it to react for 10 minutes. Rinse with tap water for 10 sec. and then stain with safranin for 1 minute. Rinse with water, blot dry and examine under oil immersion. Make a free hand sketch.
Method: II (Writz’s Method):
Staining by Ziehl’s Carbol fuchsin and Dorner’s nigrosin Solution:
Requirements:
(a) 72 hours old bacterial culture suspension.
(b) Ziehl’s Carbol fuchsin (0.3 gm. of 90% basic- fuchsin is dissolved in 10 ml. of 95% ethanol and then the volume is made up to 100 ml by adding water).
(c) Dorner’s nigrosin solution (10 gm. of nigrosin is dissolved in 100 ml distilled water and boiled for 30 minutes and then 0.5 ml. of 40% formalin is added and filtered thereafter).
(d) Distilled water.
(e) Test tube, Test tube rack.
(f) Water-bath, Burner etc.
Procedure:
About 1 ml. of bacterial suspension is taken in a test tube and then an equal quantity of carbol fuchsin is added. On a slide, one loopful of the stained preparation is mixed with one loopful of nigrosin solution. The preparation is smeared as thinly as possible and dried rapidly. The thin portion of this smear preparation is observed under microscope using oil immersion objectives.
Observation:
The background appears greyish black, the cells colourless and the endospore brilliant red (Fig. 4.3).
III. Flagella Staining:
Some eukaryotic and prokaryotic organisms can move actively by a cellular component known as flagellum which is so thin that it does not come within the resolving power of a bright field microscope. As such it requires special staining which makes it visible.
Requirements:
(i) 18 hours old slant culture of Bacillus aspiarius
(ii) Culture tube and distilled water (sterilised)
(iii) Clean, grease free slide.
(iv) Staining reagents:
Solution ‘A’:
(a) Tannic acid (10% aq. soln.) – 18 ml.
(b) FeCl3, 6H2O (6% aq. Soln.) – 6 ml.
Solution ‘B’:
(a) Soln. ‘A’ (filtrate) – 3.5 ml.
(b) Basic fuchsin (0.5% in ethanol) – 0.5 ml.
(c) HCl (concentrated) – 0.5 ml.
(d) Formalin – 2.0 ml.
Solution ‘C’:
1. (a) Basic fuchsin – 0.3 gm.
(b) Alcohol – 30 ml.
2. (a) Phenol – 5 gm.
(b) Distilled water – 95 ml.
Procedure:
1. The slant is half filled with water and kept at room temperature for about 1/2 hour without disturbance.
2. A drop of suspension is placed on the slide and held in an inclined way so that the suspension runs along the surface of the slide very slowly.
3. The slide is air dried.
4. It is stained following Bailey’s method.
(i) Solution ‘A’ is filtered and the filtrate is allowed to remain on the slide for 3 – 4 minutes.
(ii) After pouring off the solution ‘A’ from the slide, solution ‘B’ is added and is allowed to remain for 7 min.
(iii) The stained smear is washed with distilled water.
(iv) The slide is then filled with solution ‘C’ and is allowed to stand for 1 minute with gentle heat.
5. The smear is washed with water and air dried.
6. It is examined under oil immersion objective.
Observation:
Peritrichonus flagella (pink (dark) colour) attached to the surface of the red cells are observed. Some broken flagella are also visible.
Precaution:
1. Extreme care should be taken not to disturb the bacterial suspension, which may cause detachment of the flagella.
2. During staining with solution ‘C’, care should be taken not to boil directly on the flame.
IV. Capsule Staining:
Some bacterial cells are surrounded by a viscous substance forming an envelope around the cell. This structure is referred to as a capsule. This capsule takes a special type of stain and reveals its presence.
Method: I:
Requirements:
1. 24 hours old slant culture of Klebsiella.
2. Culture tube and distilled water (sterilised) for making suspension.
3. Clean, grease free slide.
4. Staining reagents:
a) 1 % aq. solution of congo red.
b) Maneval’s stain. (2 ml. of 1%-aq. solution of acid fuchsin, 4 ml. of 20% aq. solution of Ferric chloride, 10 ml. of glacial acetic acid and 30 ml. of 5% aq. solution of phenol mixed thoroughly and filtered).
Procedure:
1. An aqueous suspension of Klebsiella is prepared.
2. A drop of congo red is placed on a clean grease free slide.
3. A loopful of bacterial suspension is removed, mixed with congo red and spread gently over the slide. It is allowed to air dry.
4. The smear is flooded with Maneval’s stain and kept for at least 3 minutes.
5. The stained smear is washed with distilled water and is air dried.
6. The preparation is examined under oil immersion objective.
Observation:
The capsules are seen as unstained zones but the background is blue to blackish, and the cells arc stained red to reddish brown depending on the thickness of the smear.
Method: I:
Requirements:
The following solutions are required:
1. Crystal violet 1% aqueous solution.
2. Copper sulphate 20% aqueous solution.
Procedure:
1. A thin smear is prepared and dried in air without fixation.
2. Crystal violet is applied for 2 minutes without heating.
3. It is washed with copper sulphate solution.
4. It is then blotted, dried in air and examined under microscope.
Observation:
Capsule – pale violet, bacterial cell – deep violet.
V. Acid Fast Staining:
This is a unique staining procedure to identify some characteristic groups of bacteria termed “Acid fast bacillus”, which are extremely pathogenic to man. These bacteria belong to the genus – “Mycobacterium”.
Requirements:
1. Primary stain – Carbol fuchsin (Basic fuchsin and dilute phenol mixture).
2. Counterstain – Methylene blue (1 % aq.)
3. Acid-alcohol mixture (3% HCl or HNO3 in 95% ethyl alcohol)
4. Slide, needle, pipette, burner, blotting paper.
5. Microscope.
6. Bacterial suspension.
Procedure:
1. A bacterial smear is made on a grease free slide and then heat fixed in the usual way.
2. The smear is flooded with primary stain and kept for 3 – 5 minutes (without drying). The slain is washed with water.
3. Then the smear is washed with acid-alcohol mixture and finally counterstained with methylene blue for 2 – 3 minutes.
Observation:
Acid fast organisms stain deep red, while other non-acid fast organisms stain blue.
VI. Staining of Curd Organisms:
‘Curd’ is a milk product; it is formed due to microbiological activity in milk. The bacterium Lactobacillus produces acid in milk and then causes souring of milk and the milk are ultimately converted into curd. In addition to Lactobacillus curd also contains the bacterium Streptococcus lactis and some yeasts.
Requirements:
1. Curd sample.
2. Reagents for gram staining of curd organisms (crystal violet stain, iodine solution, safranin stain, alcohol solution).
3. Slide, needle, pipette, beaker, blotting paper.
4. Microscope, etc.
Observation:
Procedure:
A loopful of curd is placed on a clean grease free slide and diluted with 1 drop of sterilised water. A thin smear is then prepared on the slide, dried in air and finally heat fixed. The smear is stained following the usual procedure of gram staining and examined under microscope using oil immersion objective. The size, shape and gram nature of each organism is examined and recorded.