ADVERTISEMENTS:
The following points highlight the five main steps for culture of microorganisms. The steps are: 1. Preparation of Media 2. Adjustment of pH of Media 3. Preparation of Stabs and Slants 4. Pouring of Plates 5. Inoculation of Bacteria in Nutrient Slants and Agar Plates.
Step # 1. Preparation of Media:
Principle:
In preparing a culture medium for any microorganism, the primary goal is to provide a balanced mixture of the required nutrients, at concentrations that will permit good growth. No ingredient should be given in excess because many nutrients become growth inhibitory or toxic as the concentration is raised.
ADVERTISEMENTS:
A medium composed entirely of chemically defined nutrients is termed as synthetic medium. One that contains ingredients of unknown chemical composition is termed a complex medium. For different purposes in laboratory the media is used either ‘solid’ or ‘liquid’.
A. Liquid or Broth Media:
1. Nutrient Broth:
Principle:
Nutrient broth is the basis of most media used in the study of different types of microbes. It is one of the most important liquid media used for bacteriological purposes.
ADVERTISEMENTS:
Composition:
Beef extract – 3 gms. (0.3%)
Bactopeptone – 5 gms. (0.5%)
Distilled water – 1000 cc.
pH – 6.8 -7.0
Requirements:
(i) Flask – 1000 ml capacity.
(ii) Graduated cylinder – 500 ml.
(iii) Beef extract, bactopeptone, distilled water.
ADVERTISEMENTS:
(iv) Balance and weight box.
(v) pH paper, comparator block, 0.1 (N) NaOH and 0.1 (N) HCl soln.
(vi) Funnel, cotton, etc.
Procedure:
ADVERTISEMENTS:
To make 300ml of nutrient broth: –
0.9 gm. of beef extract and 1.5 gms of bactopeptone are weighed separately and taken in a conical flask. Then 300 ml dist. water is added and the ingredients are mixed thoroughly. The pH is adjusted by adding a little alkali (NaOH soln.). The flask is then plugged with cotton wool and autoclaved at 15 lbs. pressure for 15 minutes.
2. Potato-dextrose Broth:
Principle:
ADVERTISEMENTS:
It is a type of semisynthetic medium. This is very often used for the growth medium of fungi which grow better in potato-dextrose broth than in the nutrient broth.
Composition:
Freshly peeled potato – 400 gms. (40%)
Dextrose – 25 gms. (2.5%)
ADVERTISEMENTS:
Distilled water – 1000 ml.
Requirements:
(i) Flask – 250 ml.
(ii) Graduated cylinder – 250 ml.
(iii) Potato, Dextrose and distilled water.
(iv) Other requirements are as in the previous broth preparation.
ADVERTISEMENTS:
Procedure:
To make 200 ml of broth:
80 gms of freshly peeled potato and 5 gms of Dextrose are weighed and taken in a conical flask. 200 ml of dist. water is poured into the flask and the ingredients are mixed thoroughly by a glass rod. (Actually the peeled potato is boiled in flask using 100 ml water for 10 min and the extract is taken by decanting). The flask is plugged and autoclaved at 15 lbs. pressure for 15 min.
B. Solid or Agar Medium:
1. Nutrient Agar:
Principle:
Nutrient agar is an important medium for bacteriological purpose. It is simply nutrient broth solidified by the addition of agar. Due to its solid consistency, this medium serves as a good device for the culture of bacteria on a solid surface.
ADVERTISEMENTS:
Composition:
Beef extract – 3 gms. (0.3%)
Bacteriological peptone – 5 gms. (0.5%)
Agar agar – 15 gms (1.5%)
Distilled water – 1000 ml.
pH – 6.8 – 7.0
Requirements:
i) Flask – 500 ml.
ii) Graduated cylinder – 500 ml.
iii) Beef extract, bactopeptone, agar, and distilled water.
iv) Balance and weight box.
v) pH paper, comparator block 0.1(N) HCl and 0.1 (N) NaOH soln.
Procedure:
To make 500 ml of nutrient agar:
7.5 gm. of powdered agar is weighed and taken in a flask, containing 250 ml of distilled water. The content is then heated in a water-bath to allow the agar to dissolve. In another flask 1.4 gms of beef extract and 2.5 gms of peptone are dissolved in 250 ml of distilled water. The pH is then adjusted and checked with pH paper.
The two solutions are then poured in a 500 ml flask, stirred thoroughly and then heated gently. Then the medium is dispensed in culture tubes and conical flasks (as stock culture medium is autoclaved at 15 lbs. pressure for 15 min after plugging the tubes and the flask).
2. Potato-Dextrose-Agar (P.D.A.):
Principle:
Potato-dextrose-agar medium is very often used for the culture of fungi. It is simply potato dextrose broth solidified by adding agar.
Composition:
Freshly peeled potato – 400 gms. (40%)
Dextrose – 25 gms. (2.5%)
Agar-agar- 15 gms. (1.5%)
Distilled water – 1000 ml.
Requirements:
(i) Flask – 500 ml.
(ii) Graduated cylinder – 500 ml.
(iii) Beef extract, bactopeptone, agar, and distilled water.
(iv) Balance and weight box.
(v) pH paper, comparator block 0.1(N) HCl and 0.1 (N) NaOH soln.
Procedure:
To make 300 ml of P.D.A.: –
4.5 gms of agar is taken in a flask containing 150 ml of distilled water. The content is heated in a water-bath to dissolve the agar. 120 gms of freshly peeled potato is taken in a flask and 150 ml water is added to it. It is boiled for 10 mins. Then this potato extract is taken and its volume is made up to 150 ml by adding water. To this extract, 7.5 gms of Dextrose is added and thoroughly mixed.
Now the above two solutions are poured in a 500 ml flask and stirred thoroughly. This medium is dispensed in culture tubes and flasks. The tubes and flasks are plugged (Fig. 2.1.) and autoclaved.
Precautions:
(i) Agar must be liquefied properly before mixing with broth.
(ii) Agar must not be heated too much; otherwise it will lose its ability to solidify.
(iii) Dispensing should be done quickly, otherwise the agar will solidify.
A detailed list of the composition of various media is given in the Annexure.
Step # 2. Adjustment of pH of Media:
Principle:
The hydrogen-ion-concentration of culture media is of prime importance for the successful cultivation of bacteria. Some species grow best in acid medium, others in alkaline medium; still others prefer substrates neutral in reaction. The H+ concentration i.e.
pH = log (1/H+)
So, for the growth of a particular macro- organism, the medium should have a specific pH. To adjust the pH, acid and alkali are used.
Requirements:
(i) Nutrient broth
(ii) pH paper and colour standard
(iii) Comparator Block
(iv) 0.1(N) NaOH and 0.1(N) HCl soln.
(v) Pipettes and glass rod.
Procedure:
The simplest method for determining the pH of a soln. is to use commercially available pH paper which is impregnated with an indicator. The latter gives a colour change over a pH range of 6.4 to 8.2. A strip of pH paper is cut into small pieces and each piece is placed within a well on the comparator block.
With the help of a glass rod a drop of medium is drawn out and put on the piece of pH paper. The resulting colour is compared with the colour standard supplied.
The pH of the medium, if found to be acidic, is brought to the required pH by adding 0.1 (N) NaOH drop-wise and testing with pH paper after thoroughly mixing with a glass rod. Conversely, 0.1 (N) HCl is used to get an acidic pH of the medium.
Precautions:
(i) While neutralizing the broth, acid or alkali should be added drop-wise.
(ii) After adding acid or alkali the medium should be stirred to ensure proper mixing of the acid or alkali added.
(iii) At every step colour reaction should be noted.
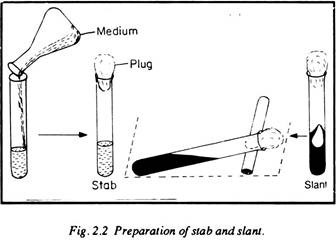
Step # 3. Preparation of Stabs and Slants:
Procedure:
In order to prepare stabs, the medium is poured up to 1/2 of the culture tube (about 20 ml), which is then plugged carefully and sterilised in autoclave. After sterilization, the culture tube is kept erect in a test tube stand until the medium solidifies. Then they are collected in a wire-net basket and preserved.
In order to prepare “slants” the medium is taken up to 1/4 of a culture tube (about 7 ml) with the help of a measuring cylinder and funnel. Then the culture tubes are plugged and autoclaved. After sterilisation, before the medium sets, the tubes are sloped on a bench by leaning them against a length of a wooden stick of 1/2″ thickness in such a fashion that the medium does not touch the plug.
The culture tubes are maintained in that position until the medium solidifies. After solidification of the medium, the “slants” are collected in a wire net basket and preserved with a label indicating the date of preparation and the nature of the medium (Fig. 2.2).
Precautions:
(i) Culture tubes should be plugged with non-absorbent cotton.
(ii) It should be noted that during dispensing, the medium does not stick to the sides of the culture tubes.
(iii) During slanting, care should be taken to see that the medium does not touch the plug.
(iv) Stabbing or slanting should be done just before solidification i.e. at around 47°C, otherwise, there will be water inside the slant.
(v) Stabs and slants should not be disturbed before the medium solidifies.
Step # 4. Pouring of Plates:
Principle:
Plate culture consists of an organism growing on a solid medium contained in a petridish. “Pouring of plates” refers to the process of pouring melted nutrient agar into petridishes. By this process a larger surface area is created for the growth of microbes in all directions.
Requirements:
(i) Nutrient agar “stab” or medium in flask.
(ii) Sterilised petridishes.
(iii) Burner, water-bath.
(iv) Absorbent cotton, rectified spirit, glass marking pencil etc.
Procedure:
Solid medium contained in culture tubes or flasks is melted in a water-bath. Once the agar is melted perfectly, the medium is allowed to cool around 45°C. The working table is cleaned and sterilised with cotton soaked in rectified spirit. Hands are also sterilised with rectified spirit.
The cotton plug of the melted tube or flask is opened near a flame and the mouth of the tube/flask is flamed in a semi-horizontal position. With the left hand the lid of a petridish is raised far enough to permit the mouth of the tube or flask to enter without touching the sides.
About 15 ml medium is quickly and carefully poured into the petridish, the tube/flask is withdrawn and the cover or lid is replaced. The petridish is tilted slightly with the movement of the wrist to allow homogeneous spreading of the medium (Fig. 2.3). When the medium solidifies, the plates are incubated at 30°C in an inverted position i.e. upside down so that moisture drop does not fall on the medium.
Precautions:
(i) All work should be done aseptically.
(ii) After solidification the plates should be kept inverted.
(iii) During pouring it should be noted that the mouth of the tube/flask does not touch any part of the petridish.
Step # 5. Inoculation of Bacteria in Nutrient Slants and Agar Plates:
Materials Required:
(i) Nutrient slants and nutrient agar plates.
(ii) Inoculating needle.
(iii) Burner.
(iv) Bacterial culture slant:
(a) Escherichia coli,
(b) Bacillus subtilis,
(c) Klebsiella pneumoniae,
(d) Staphylococcus aureus.
Method:
First the inoculating needle (Fig. 2.4) is sterilised by heating strongly in a flame and cooled by holding it outside the flame. After cooling the needle further by touching it on the ‘extra’ medium (where there is no growth) in supplied slants, a very small amount of inoculum is taken out by means of the needle and then inoculates in the previously prepared slants in a zigzag manner.
Each bacterial culture is inoculated in duplicate. The needle is then flamed to ensure killing of bacteria in the needle. (Fig. 2.5).
For streaking plates, inoculum is taken out in the same way as mentioned and touched on the solid medium in a plate. The needle is flamed to kill excess bacteria and cooled. The inoculum is spread by streaking a line with the needle (Fig. 2.6). The needle is again burnt for the same purpose and cooled.
Continuous and zigzag lines arc streaked so as to reduce gradually the number of cells along the line and to get isolated single colonies after incubation. The whole operation of inoculation is performed aseptically in front of a strong flame. The inoculated slants and plates (in inverted position) are incubated overnight at 37°C.
Observation:
In slants:
Heavy growth is found along the lines of inoculation.
In plates:
Heavy growth is noted where inoculation was made first but growth gradually decreases (i.e. only a few isolated single colonies are grown) in regions away from inoculation site.