ADVERTISEMENTS:
In this article we will discuss about:- 1. Meaning of Xenobiotic Compounds 2. Types of Recalcitrant Xenobiotic Compounds 3. Hazards 4. General Features of Biodegradation 5. Co-Metabolism and Gratuitous Metabolism 6. Biodegradation of Halogenated Compounds 7. The Origin of Capacity to Degrade Xenobiotics.
Meaning of Xenobiotic Compounds:
Xenobiotic compounds are man-made chemicals that are present in the environment at unnaturally high concentrations. The xenobiotic compounds are either not produced naturally, or are produced at much lower concentrations than man. Microorganism have the capability of degrading all naturally occurring compounds; this is known as the principle of microbial infallibility proposed by Alexander in 1965.
Microorganisms are also able to degrade many of the xenobiotic compounds, but they are unable to degrade many others. The compounds that resist biodegradation and thereby persists in the environment are called recalcitrant.
ADVERTISEMENTS:
The xenobiotic compounds may be recalcitrant due to one or more of the following reasons:
(i) They are not recognised as substrate by the existing degradative enzymes,
(ii) They are highly stable, i.e., chemically and biologically inert due to the presence of substitution groups like halogens, nitro-, sulphonate, amino-, methoxy- and carbamyl groups,
(iii) They are insoluble in water, or are adsorbed to external matrices like soil,
ADVERTISEMENTS:
(iv) They are highly foxic or give rise to toxic products due to microbial activity,
(v) Their large molecular size prevents entry into microbial cells,
(vi) Inability of the compounds to induce the synthesis of degrading enzymes, and
(vii) Jack of the perm-ease needed for their transport into the microbial cells.
Types of Recalcitrant Xenobiotic Compounds:
The recalcitrant xenobiotic compounds can be grouped into the following 6 types:
(i) Halocarbons,
(ii) Polychlorinated biphenyls,
(iii) Synthetic polymers,
(iv) Alkylbenzyl sulphonates,
ADVERTISEMENTS:
(v) Oil mixture and
(vi) Others.
The structural features that make these compounds resistant to microbial degradation include the following:
(i) Presence of halogens in the place of hydrogen in the molecule; the carbon-halogen bond is highly stable and its cleavage requires considerable energy,
ADVERTISEMENTS:
(ii) Substitution of H by other groups like nitro-, sulphonate, methoxy-, amino- and carbomyl groups,
(iii) Cyclic structures, aromatic compounds, cycloalkanes and heterocyclic compounds are more recalcitrant than linear chain or aliphatic compounds,
(iv) Branched linear chains resist biodegradation etc.
In general, the more complex is the structure of a xenobiotic compound, the more resistant it is to biodegradation. Many other xenobiotics resist biodegradation due to their large molecular size and insolubility in water.
ADVERTISEMENTS:
(i) Halocarbons:
These compounds contain different numbers of halogen (e.g., CI, Br, F (fluorine), I) atoms in the place of H atoms. They are used as solvents (chloroform, CHCI3), as propellants in spray cans of cosmetics, paints etc., in condenser units of cooling systems (Freons, CCI3F, CCl2F2, CClF3, CF4), and as insecticides (DDT, BHC, lindane etc.) and herbicides (dalapon, 2, 4-D, 2, 4, 5-T etc.).
The C1-C2 haloalkanes like chloroform, freons etc. are volatile and escape into the atmosphere where they destroy the protective ozone (O3) layer leading to increased UV radiation. Pesticides (herbicides, fungicides and insecticides) are applied to crops from where they leach into water bodies; many of them are subject to bio-magnification.
(ii) Poly chlorinated Biphenyls (PCB’s):
ADVERTISEMENTS:
These compounds have two covalently linked benzene rings having halogens substituting for H. PCB’s are used as plasticisers, insulator coolants in transformers and as heat exchange fluids. They are both biologically and chemically inert to various degrees, which increases with the number of chlorine atoms present in the molecule.
The recalcitrant nature of the above two groups of compounds is due to their halogenation and as well their cyclic structure (PCB’s).
(iii) Synthetic Polymers:
These compounds are produced as plastics, e.g., polyethylene, polystyrene, polyvinyl chloride etc., and nylons which are used as garments, wrapping materials etc. They are recalcitrant mainly due to their insolubility in water and molecular size.
(iv) Alkylbenzyl Sulfonates:
These are surface-active detergents superior to soaps. The sulphonate (— SO3–) group present at one end resists microbial degradation, while the other end (non-polar alkyl end) becomes recalcitrant if its is branched, (resistance increases with the degree of branching). At present, alkylbenzyl sulphonates having non-branched alkyl ends are used; these are biodegraded by β-oxidation from their alkyl ends.
ADVERTISEMENTS:
(v) Oil Mixtures:
Oil is a natural product, has many components and is biodegradable, the different components being degraded at different rates. Biodegradation is able to handle small oil seepages. But when large spills occur the problem of pollution becomes acute. Oil is recalcitrant mainly because of its insolubility in water and due to the toxicity of some of its components.
(vi) Other Xenobiotic Compounds:
A number of pesticides are based on aliphatic, cyclic ring structures containing substitution of nitro-, sulphonate, methoxy-, amino- and carbomyl groups; in addition, they also contain halogens. These substitutions make them recalcitrant.
Hazards from Xenobiotic Compounds:
The xenobiotics present a number of potential hazards to man and the environment which are briefly listed below.
(i) Toxicity:
ADVERTISEMENTS:
Many xenobiotics like halogenated and aromatic hycrocarbons are toxic to bacteria, lower eukaryotes and even humans. At low concentrations they may cause various skin problems and reduce reproductive potential.
(ii) Carcinogenicity:
Certain halogenated hydrocarbons have been shown to be carcinogenic.
(iii) Many xenobiotics are recalcitrant and persist in the environment so that there is a build up in their concentration with time.
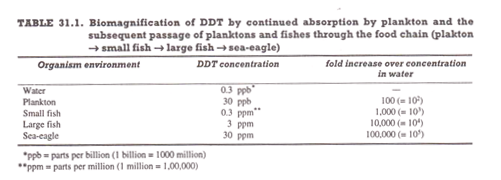
(iv) Many xenobiotics including DDT and PCB’s are recalcitrant and lipophilic; as a consequence they show bioaccumulation or bio-magnification often by a factor of 104 – 106.
Bio-magnification occurs mainly because of the following two reasons:
(i) These compounds are continuously taken up from the environment and accumulated in the lipid deposits of body, e.g., a 100-fold accumulation of DDT by plankton from water,
(ii) Such organisms are consumed by other organisms in a sequential manner constituting the food chain, e.g., plankton → small fish → large fish → sea-eagles; the concentration of xenobiotics builds up as we move up in the food chain (Table 32.1).
In case of DDT a 105 – fold increase occurs in sea-eagles as compared to the concentration present in the aqueous environment as a result of which sea-eagles laid fragile eggs. DDT and PCB’s have been found in human tissues in high but sub-lethal concentrations in those countries where they have been used, although humans were often not in direct contact with these chemicals.
(v) They are produced and used in large quantities which favours their accumulation in nature.
General Features of Biodegradation of Xenobiotics:
Since xenobiotics consist of a wide variety of compounds, their degradation occurs via a large number of metabolic pathways.
Degradation of alkanes and aromatic hydrocarbons generally occurs as follows:
(i) An oxygenase first introduces a hydroxyl group to make the compound reactive,
(ii) The hydroxyl group is then oxidised to a carboxyl group,
(iii) The ring structure is opened up (in case of cyclic compounds),
(iv) The linear molecule is degraded by β-oxidation to yield acetyl CoA which is metabolised in the usual manner. For example, an n-alkane is oxidised as follows.
Similarly, an alicyclic hydrocarbon, e.g., cyclohexane, is oxidised as follows:
(i) First an oxygenase adds an —OH group in the ring,
(ii) Then another oxygenase forms an ester in the form of a lactone,
(iii) Which is then hydrolysed to open the ring structure to yield a linear molecule (Fig. 32.12).
In both these oxidations mono-oxygenases are involved which add oxygen to a single position in the molecule. In contrast, oxidation of benzene ring may involve a di-oxygenase which adds oxygen at two positions in the molecule in a single step (Fig. 32.13).
Both mono- and di-oxygenases are of a variety of types: some react best with short chain alkanes, while others act on cyclic alkanes. But these enzymes are not very specific and each enzyme oxidise a limited range of compounds. Thus xenobiotics are degraded by a wide variety of microorganisms, each of which degrades a small range of compounds.
Frequently, oxidation of xenobiotics involves cytochrome P450 or rebredoxin. In addition, the halogens and/or other substituent groups are either modified or removed usually as one of the initial reactions or sometimes it is achieved later in the process.
Hydrocarbon Degradation:
The degradation of hydrocarbons is briefly outline below:
(i) Halomethanes:
Halomethanes are transferred into methanol by the enzyme methane mono-oxygenase which uses them as substrate; this enzyme occurs in a number of methylotrophs. Alternatively, a glutathione-dependent hydrolase catalyses oxidative dechlorination of halomethanes into methanol; this reaction is anaerobic and uses oxygen derived from water. Methanol is oxidised to CO2 + H2O via formaldehyde and formic acid.
(ii) Cyanide:
Cyanide(HCN) is toxic to biological systems and even microorganisms capable of degrading cyanide cannot withstand a high concentration of HCN. Some of the cyanides, e.g., HCN and CH3CN, are volatile. Therefore, disposal of cyanide is strictly controlled.
It is degraded as follows:
(iii) Aliphatic Hydrocarbons:
Aliphatic Hydrocarbons may be saturated or unsaturated, n-Alkanes of 10-24 carbons are the most readily biodegraded. Similarly saturated aliphatics are easier to degrade than unsaturated ones and branched chains show decreased biodegradation. Biodegradation of n-alkanes is catalysed by oxygenases to produce carboxylic acid, which is then degraded by β-oxidation.
Oxidation may involve the methane group at one end of n-alkane molecule, or it may occur at a β-methylene group (Fig. 32.14). Sometimes, both terminal methyl groups are oxidised to yield a dicarboxylic acid; this reaction is used by many microorganisms for the biodegradation of branched chain n-alkanes.
(iv) Alicyclic Hydrocarbons:
Alicyclic hydrocarbons are present naturally in waxes from plants, crude oil, microbial lipids etc. and are represented by xenobiotics used as pesticides and also in petroleum products. Un-substituted cyclohexane rings are oxidised as outlines in Fig. 32.12.
(v) Aromatic Hydrocarbons:
Aromatic hydrocarbons are rather stable.
These are oxidised by di-oxygenascs (Fig. 32.13) to catechol which is further metabolised by two separate pathways:
(i) In case of ortho-ring cleavage pathway, a 1, 2- dioxygenase cleaves the ring between the two adjacent hydroxyl groups and sequential catabolism of the product cis, cis-muconate yields succinate + acetyl CoA (Fig. 32.13).
(ii) Alternatively, the enzyme 2, 3-di- oxygenase cleaves the ring between the carbon atom having an OH group and an adjacent carbon lacking an OH group (meta-cleavage); the products at the end of reaction sequence are acetaldehyde and pyruvate. Both ortho and meta pathways arc involved in degradation of aromatic hydrocarbons. Benzene is degraded by the meta pathway.
(vi) Polycyclic Hydrocarbons:
Polycyclic hydrocarbons contain two or more rings. Generally, one of the terminal rings is attacked by a di-oxygenase, leading to ring cleavage and degradation so that in the end a single ring remains which is catabolised in a manner similar to that described above (Fig. 32.13).
Degradation of complex molecules containing aliphatic, aromatic, alicyclic or heterocyclic components is difficult to generalise but the following features are observed:
(i) Amide-, ester- or ether bonds are first attacked and further degradation of the products so generated takes place;
(ii) If these bonds are absent or inaccessible; aliphatic chains are degraded;
(iii) If aliphatic chains are branched, the aromatic component of complex molecules may be attacked,
(iv) The site and mode of attack depends on the molecular structure, the microorganism involved and the environmental conditions,
(v) In general, recalcitrance of various benzene derivatives increases with the substituent groups (at meta position) as follows: COOH = OH < -NH2 < -O—CH3 < -SO3– < -NO2–.
Further, the greater the number of substituent groups on the benzene ring, the higher the degree of recalcitrance. (ii) The position of substitution also affects recalcitrance as meta > ortho > para in recalcitrance (Fig. 32.15).
Co-Metabolism and Gratuitous Metabolism of Xenobiotic Compounds:
Some xenobiotic compounds, e.g., cyclohexane, halogenated compounds etc., are degraded by microbes, but these compounds are rarely, if ever, used as sources of energy and carbon by them.
Degradation of such compounds, therefore, depends on the presence of another compound which induces the necessary enzymes, and metabolism of which provides both energy and reducing equivalents for the degradation of xenobiotic compounds (and the C, energy etc. needed for microbial growth).
Clearly in such cases, degradation of xenobiotic compounds depends on the presence and metabolism of a suitable substrate called co-metabolite, such a degradation is called co-metabolism.
In contrast, several xenobiotic compounds are degraded by an existing pathway and are used by microbes as sources of energy and reducing equivalents; this is known as gratuitous metabolism. In such a metabolism, the necessary enzymes are already induced by another compound which is not needed as a co-metabolite.
The xenobiotics degraded by both gratuitous and co-metabolism are very similar to the natural substrates of the enzymes involved in their degradation. Often a xenobiotic compound may not be completely degraded by gratuitous metabolism, but the product may be less polluting or may be used as substrate by some organisms.
Biodegradation of Halogenated Compounds:
Biodegradation of such compounds involves two distinct steps:
(i) Elimination of the halogen groups, and
(ii) Degradation of the non-halogenated product molecule.
Removal of halogen molecule may occur either directly involving the removal of hydrogen halide (e.g., HCI, Fig. 32.16), or it may involve the substitution of halogen by —H (Fig. 32.16B), -OH (Fig. 32.16C) or a -thio group (Fig. 32.16D).
The direct halogen removal produces a double bond and is relatively rate in nature. The mechanism involving halogen substitution, especially by —OH, is far more common particularly for fully reduced aliphatics or aromatics.
The aerobic degradation of halogenated aromatic compounds usually involves the following steps:
(i) Addition of —OH group by a dioxygenase to yield chlorinated catechol’s (as in case of normal benzene; Fig. 32.13),
(ii) Cleavage of the ring by ortlio or meta cleavage (meta cleavage often results in the halogen not being eliminated from the aliphatic product which may lead to an accumulation of toxic metabolites),
(iii) Elimination of the halogen from the straight chain (alophatic) product, and finally,
(iv) Degradation of the aliphatic hydrocarbon (non-halogenated) so produced. In case of phenols (which already have one —OH group), the step 1 reaction is catalysed by a hydroxylase which adds another —OH group to yield the catechols.
Rarely, however, the halogen may be removed before cleavage of the aromatic ring. Usually, the removal of halogen’ occurs by its substitution with a —H or —OH group; the resulting non-halogenated aromatic compound is then metabolized as depicted in Fig. 32.13.
The Origin of Capacity to Degrade Xenobiotics:
Continued exposure of microorganisms to xenobiotic compounds can often lead to the evolution of metabolic processes needed to wholly or partly degrade the xenobiotic.
These capabilities may arise due to:
(i) Mutation, and
(ii) Transfer of plasmid borne genes.
Gene mutation occurs spontaneously at a low frequency. But the short generation time and very large populations of microorganisms make mutation a potent source of genetic variation.
Mutations can be expected to either modify the active site of an enzyme so that it has an increased affinity for the xenobiotic, or it can eliminate regulatory controls and enhance its production. Such changes only enhance the rate of degradation of a xenobiotic; they rarely, if ever, generate a new enzyme function.
Often new enzyme activities are acquired by plasmid transfer (usually through conjugation) since many of the key enzymes concerned with xenobiotic metabolism are plasmid borne. Most bacteria containing such plasmids are gram-negative aerobes mainly from the genes Pseudomonas. Some plasmids encode the entire pathway for xenobiotic degradation, e.g., TOL plasmids encode the enzymes for toluene degradation.
But many plasmids encode only some of the degradative enzymes, e.g., pAC21 for p-chlorobiphenyl degradation and pAC25 for 3-chlorobenzoate degradation; in such cases, the remaining enzymes involved in the degradation must be provided either by the chromosomal genes of the cell or by another microorganism.
Plasmid transfers allow a microorganism:
(i) To acquire the genes needed to complete the pathway for a xenobiotic metabolism, and/or
(ii) To gain genes which improves the rate and/or the nature of degradation.
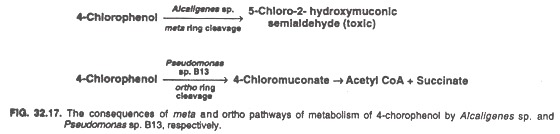
The ability of plasmid transfer can be exploited to create microorganisms with novel characteristics. For example, Alcaligenes sp. degrade 4-chlorophenol to 5-chloro-2-hydroxymuconic semialdehyde (by meta cleavage of the ring) which is toxic.
When the concentration of 4-chlorophenol is higher than 4 m mol/1, the level of this intermediate becomes toxic preventing its further degradation. In contrast Pseudomonas strain B13 has a plasmid-borne gene which encodes the enzyme 1, 2-di-oxygenase; this enzyme cleaves 4-chlorophenol by ortho pathway which avoids the production of toxic intermediates (Fig. 32.17).
When a mixture of Alcaligenes sp. and Pseudomonas strain B13 were maintained in laboratory, the Alcaligenes sp. acquired the plasmid and the ability for ortho ring cleavage from Pseudomonas strain B13. This is an example of the production of a natural recombinant species of bacteria. Attempts are being made to develop genetically engineered bacteria having recombinant pathways for xenobiotic compound degradation.
The strategies most likely to succeed are:
(i) To transfer genes encoding enzymes with a wider specificity for substrates, and
(ii) To modify regulatory elements with a view to enhance enzyme synthesis, to promote assimilation of the xenobiotic compound into the cell etc.
The possibilities of creating a ‘super bug’ capable of degrading a wide variety of xenobiotic compounds under a number of conditions in stable association with native microorganisms seems unrealistic.
This is because microorganisms carrying additional recombinant DNA are poor competitors against native microorganisms. In addition, the release of genetically engineered microbes (GEMs) in the environment is illegal in many countries.
Use of Mixed Microbial Populations:
The use of mixed populations of microbes for degradation of xenobiotic compounds is desirable due to the following reasons:
(i) Two different microbes can together degrade degrade a xenobiotic completely, while either of them alone is incapable of this feat. In such a case, the product of degradation by one microorganism serves as the substrate for the other.
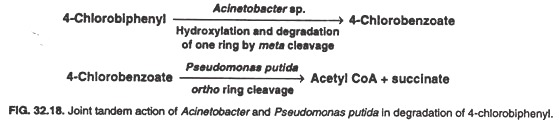
For example, Acinetobacter sp. has plasmid-borne genes for dihydroxylation of one of the rings of 4-chlorobiphenyl, its meta ring cleavage and subsequent degradation to produce 4-chlorobenzoate; however, it can not degrade this product any further.
A Pseudomonas putida strain cleaves the ring of 4-chlorobenzoate by ortho pathway, to ultimately generate acetyl-CoA and succinate; but this bacterium can not utilize 4-chlorobiphenyl.’ Thus Acinetobacter and P. putida act in tandem to degrade completely the xenobiotic 4-chlorobiphenyl which neither of them can accomplish on its own (Fig. 32.18).
(ii) One microorganism may produce growth factor/nutrient required by the other. For example, Nocardia sp. degrades cyclohexane but is unable to produce biotin. A Pseudomonas sp. strain produces biotin but can not degrade cyclohexane. Nocardia sp. breakdown cyclohexane; the breakdown products and Nocardia cell lysis products are used by Pseudomonas strain which grows and releases biotin.
The biotin, in turn, promotes Nocardia growth and cyclohexane breakdown. Thus a mixture of these two strains would breakdown cyclohexane but neither of them can do it alone.
(iii) Co-culture may lead to plasmid transfer into a faster growing species thereby creating a faster growing species capable of degrading the xenobiotic. An example is the transfer of plasmid from Pseudomonas sp. strain B13 into Alcaligenes sp. which is faster growing than Pseudomonas sp. B13.
(iv) In natural environments, mixtures of xenobiotic compounds are found. Use of mixed cultures increases the likelihood of the microbial components of mixed culture being able to degrade all the xenobiotic compounds present.
(v) The biological treatment system, i.e., the microbial community used for degrading xenobiotics, is more stable and can withstand occasional shock loadings.
(vi) Biodegradation rates are usually higher due to the microbial interactions described above.
Practical Approaches to Xenobiotic Degradation:
Biodegradation of xenobiotic compounds depends on their concentration (too high concentration may be toxic), pH of the medium, temperature, availability of water and other nutrients and presence of organic compounds (these may be co-metabolites, inhibitors or preferred substrate, in place of the xenobiotic, by microorganisms). In general, the xenobiotic compound should be available in an acceptable concentration and toxic levels should not occur.
ADVERTISEMENTS:
In a treatment system, a constant supply of the compound should be available for selective maintenance of microbes capable of its degradation. In addition, interfering organic compounds should not be present in the environment.
Practical application of microbes for xenobiotic degradation is facilitated by:
(i) Supply of sufficient nutrients or co-metabolites,
(ii) Maintenance of the xenobiotic compounds to non-toxic levels and
(iii) Provision of microbial population or inoculum.
(i) Nutrients and Co-metabolites:
All the nutrients required for growth and metabolism of microbial cells must be available in the solution containing the xenobiotic compound. In general, nitrogen,- phosphorus and, sometimes, sulphur may have to be added to the solution.
Where necessary, adequate 02 supply should be ensured by aeration of the medium since xenobiotic compounds are degraded aerobically. In addition, some compounds may be required as co-metabolites to induce the necessary enzymes and to provide the energy and the reducing equivalents for xenobiotic degradation.
(ii) Xenobiotic Concentration:
In case the xenobiotic concentration is high, it should be reduced by appropriate dilution with, usually, water. In continuous treatment system, e.g., for effluents, the amount of xenobiotic entering the system should not exceed the amount being degraded by the system.
At the same time, xenobiotic should always to present in the effluent in order to maintain the necessary microbial population. However, when the concentration of the xenobiotic compound is relatively low, a higher density of the degradative microbes will be needed for degradation of the compound.
(iii) Microbial Inoculum:
In a treatment system natural evolution of the microbial population will take time. Therefore, it is customary to inoculate the system with a suitable population of microbes having the necessary degradative capabilities; this is called bio-augmentation. A number of inoculants designed to degrade various xenobiotic compounds are commercially available.
These inoculants consist of naturally occurring microbial species which have been isolated, purified and characterised for various features, including their xenobiotic degradation capabilities. The stocks of various isolates are preserved by air-drying or freeze-drying techniques.
The commercial inoculum is prepared by selecting the strains needed for degradation of the specified xenobiotic compounds, and then matching and blending them; bulking agents, dispersant chemicals, wetting agents and nutrients are also added. The inoculants are usually available as dry powders which are easy to store, transport and reconstitute at site (by simply adding water).