ADVERTISEMENTS:
The following points highlight the top three experiments on environmental microbiology. The experiments are: 1. Bacteriological Test for Water Pollution Analysis 2. Membrane-Filter Technique for Bacteriological Examination of Water 3.Multiple Tube Method for Enumeration of Bacteria.
Environmental Microbiology: Test # 1.
Bacteriological Test for Water Pollution Analysis:
From bacteriological standpoint, water contains a number of pathogens along with many forms of non-pathogenic organisms. Normally, pathogens are likely to gain entrance into water bodies sporadically, and they do not survive for long periods of time. It is known that the pathogens that gain entrance into bodies of water arrive there via intestinal discharges of humans and animals.
ADVERTISEMENTS:
Furthermore, certain bacterial species, particularly E. coli and related organisms designated as coliforms, faecal streptococci (S.faecalis) and Clostridium per-fringes, are normal inhabitants of the large intestine of humans and other animals and are consequently present in faeces. Thus, the presence of any of these bacterial species in water is evidence of faecal pollution of human or animal origin.
The coliform group of bacteria includes all the aerobic and facultatively gram-negative, non-sporulating bacilli that produce acid and gas from fermentation of lactose. The classical species of this group are E. coli and Enterobacter aero-genes. These organisms are also related to other enteric genera viz. Salmonella, Shigella, Klebsiella, Proteus, Serratia, etc. gram-negative and non-sporulating forms.
General’ scheme of laboratory testing for detection of coliform group in water is given below:
Fermentation of lactose broth and demonstration of gram-negative, non-sporulating bacilli constitute a positive completed test demonstrating the presence of some members of the coliform group in the sample examined.
The standard microbiological techniques involve successive steps:
(1) The Presumptive test,
(2) The confirmatory test and
(3) The complete test.
Requirements:
1. Water samples.
2. Lactose broth (Beef extract – 3 gm., Peptone – 5 gm., Lactose – 10 gm., Distilled water – 1 lit., pH – 6.8 to 7.8) in sterilised culture tubes.
3. Sterilised petridishes,
ADVERTISEMENTS:
4. Pipette, Incubators, etc.
Procedure:
I. Preparation of the sample:
The water sample is shaken vigorously 25 times and 1:10, 1: 100, 1: 1,000 distributions are made.
ADVERTISEMENTS:
II. Presumptive test for coliform bacteria:
1 ml. of diluted water sample is incubated in Lactose broth containing culture tubes at 37°C for one day. Each tube has Durham’s vial. The tubes are then tested for gas and acid production.
Observation:
The presence of gas indicates the positive presumptive test.
ADVERTISEMENTS:
III. Confirmatory test for Coliform bacteria:
This test is necessary only when the test samples show positive presumptive test. For this test, streaking is done on an eosin methylene blue agar plate (Peptone – 10 g., Lactose – 5 g., Sucrose – 5 g., K2HPO4 – 2 g., Agar – 13.5 g., Eosin Y – 0.4 g., Methylene blue – 0.065 g., Water – 1 lit.), with the inoculum obtained from each of the lactose broth cultures showing production of gas. The plates are then incubated at 35 ± 1°C for 24 hours.
Observation:
If typical colonies develop, the confirmatory test may be considered positive. (characteristics indicated below).
ADVERTISEMENTS:
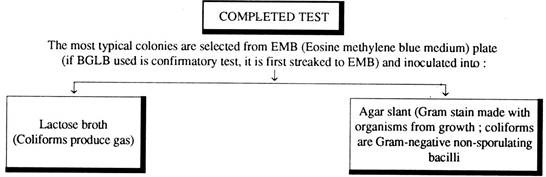
IV. Completed test for Coliform bacteria:
A few isolated colonies arc picked up (and they are likely to be coliform groups) from each of the EMB plates and each one is incubated on a lactose broth fermentation tube and a nutrient agar slope. Then the tubes are incubated at 35 ± 1°C for 1 – 2 days. Finally the gas production capacity of these organisms on lactose broth is observed and gram stained preparation is made from nutrient agar slopes.
Observation:
The production of gas and observation of gram-negative non-spore forming rod-shaped bacteria in the nutrient agar media is satisfactory completed test for demonstrating the presence of coliform bacteria in the test samples. The presence of one such bacterium per 100 ml. of water makes the water polluted and it is not fit for drinking.
Precautions:
1. The sample must be collected aseptically and carried to the laboratory in a sterilised bottle.
ADVERTISEMENTS:
2. The sample must be a representative sample.
3. Contamination must be avoided during and after sampling.
4. Testing should not be delayed.
[In case of unavoidable delay, the sample must be stored at a temperature between 0 to 10°C.]
Environmental Microbiology: Test # 2.
Membrane-Filter Technique for Bacteriological Examination of Water:
A sterilised disc is placed in a filtration unit. A volume of the water to be tested is drawn through this disc; the bacteria are retained on the surface of the membrane. Then the filter disc is removed and placed on an absorbent pad that has previously been saturated with the appropriate medium.
ADVERTISEMENTS:
Alternately, the disc can be placed on the surface of an agar medium in a petridish. On incubation, colonies will develop on the filter disc wherever bacteria were entrapped during filtration process.
The membrane filter technique has several desirable features:
1. A large volume of water sample can be examined. Theoretically, almost any volume of non-turbid water can be filtered through the disc, the organisms from any given volume being deposited on the disc.
2. The membrane can be transferred from one medium to another for purpose of selection or differentiation of organisms.
3. Results can be obtained more rapidly than by the conventional MPN standard methods.
4. Quantitative estimation of certain bacterial types, e.g., coliforms, can be accomplished when appropriate media are used.
Requirements:
1. Millipore filtration apparatus set,
2. Sterilised disc, forceps, pipette and petridish,
3. Enrichment broth (Yeast extract – 6 g., Peptone – 40 g., K2HPO4 – 3 g., NaCl – 5 g., Distilled water – 1 lit., pH – 7),
4. Endo-medium broth (Peptone – 10 g., Lactose – 10 g., K2HPO4 – 3.5 g., Sodium sulphite – 2.5 g., Basic fuchsin – 0.5 g., Distilled water – 1 lit.),
5. Brilliant green Lactose bile broth (Peptone – 10 g., Lactose – 10 g., Oxgall – 20 g., Brilliant green – 133 mg. Distilled water – 1 lit.)
6. Reagents for biochemical tests.
Procedure:
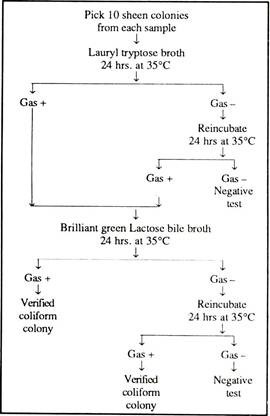
Approximately 1 ml. of Endo-medium is added to the pad contained in the disc. The disc is then covered until the water sample has been filtered through the membrane. The filter is placed on a filter holder and clamped in position below the funnel, and the water sample (100 ml. for testing portability) is poured into the funnel and passed through the Millipore filter with the aid of a vacuum pump.
The funnel is removed, and the filter disc, handled with sterilised forceps, placed on the pad previously impregnated with medium. The plates are incubated at 35°C for 20 hrs. at which time the number of coliform colonies can be determined. Now the bacteria are seen as “sheen” colonies. For further verification of the identity of the coliform colonies the following biochemical tests can be made:
In general, gas production is considered to be the confirmatory test for coliform bacteria. Normally, this technique, with modifications, has been adopted for many microbiological procedures other than the examination of water.
The detailed scheme is given in Fig. 11.1.
Environmental Microbiology: Test # 3.
Multiple Tube Method for Enumeration of Bacteria:
Principle:
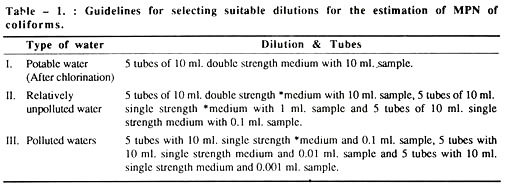
The most probable number (MPN) concept for estimating bacteria is used on “multiple dilution to extinction” i.e. subdivision of the sample and is valuable for estimating the population of microorganisms where other bacterial species predominate, e.g. in contaminated water.
The MPN method is most frequently used for determining coliform counts but specific types of other organisms can also be enumerated e.g. Salmonella, Staphylococcus, Streptococcus, Clostridium, Vibrio, Klebsiella or Aero-monas, by using appropriate selective broth and media.
Requirements:
1. MacConkey Broth and MacConkey medium (Peptone – 17 gm., Proteose peptone – 3 gm., Lactose – 10 gm., Bile salt – 1.5 gm., NaCl – 5 gm., Neutral red – 3 ml. Agar – 15 gm., Distilled water – 1 lit.)
2. Brilliant green bile salt Lactose broth (BGBB)
3. Peptone water (Peptone – 10 gm., NaCl – 5 gm., Distilled water – 1 lit.)
4. Kovacs reagent (Para dimethyl amino Benz aldehyde-5 g., dissolved in 100 ml. mixture of conc. HCl and N-amyl alcohol in 1: 3 ratio).
5. Ringers solution (NaCl-22.5 gm., KCl-0.105 g/lit. CaCl2 – 0.12 gin. ; NaHCO3 – 0.15 gm., Distilled water – 1 lit.)
6. Autoclave, Hot air oven, Incubator, Water bath, Microscope.
7. Test tube, Durham’s tubes, Pipettes, Water samples, Sterilized distilled water.
Procedure:
Water sample is collected aseptically in a sterilised bottle and then the sample is vigorously inverted rapidly several times to ensure that the bacteria are homogeneously distributed in the sample. Then a serial dilution of the water sample up to 10-2, 10-3 or 10-4 dilution is prepared.
Now, 1 ml. of diluted samples is placed in a sterilised culture tube containing 5 ml. medium broth and incubated at 37°C for 24 hrs. Thereafter, each positive tube is sub-cultured in 5 ml. of MacConkey broth or 5 ml. of Brilliant green bile broth.
The negative types are also re-incubated in the above stated media for 24 hours. Finally the total number of positive tubes is recorded and the presumptive coliform count/100 ml of sample is calculated. At the same time, in MacConkey broth, the gas production efficiency of the organisms is tested and thus the confirmed coliform count is calculated.
Identically, the gas and indole production efficiency of the bacteria in BGBB medium is tested for the presence of FAECAL COLIFORM (confirmative test) and also for quantitative estimation.
For final and complete confirmation, BGBB positive samples are sub-cultured in MacConkey medium and incubated at 37°C for 18 hours. Then typical pink coliform colonies will appear. This is followed by gram staining of colonies which show gram negative red rods.
A standard method is given in Fig. 11.2.
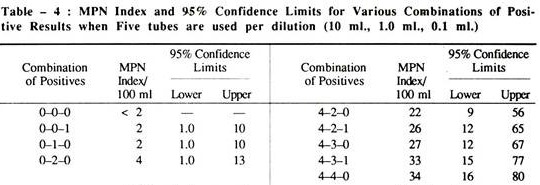
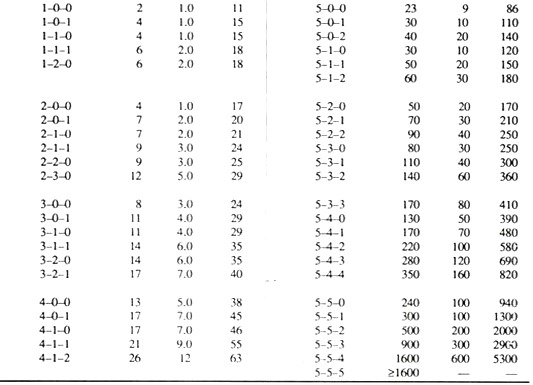
Calculation of MPN:
The smallest sample volume in which all replicate tubes are gas positive is selected as the starting dilution. The results of this test volume and of the next two smaller volumes are used to determine the positive tube combination. The MPN tables are usually limited to values for test starting with 10 ml. sample portions; hence the following adjustments have to be made.
MPN table value (10 starting dilution) = MPN.
For instance,
In a positive tube combination result of 5-3-0, where the smallest sample equals 0.01 ml., the MPN tube gives a value of 79.
MPN then equals: 79 (10/0.01) = 79,000 coliform/100 ml.
N.B. When none of the dilutions used, starting with 1 ml. volume gives a positive result, then MPN is ∠20/100 ml.
When all tubes are positive at a starting point of 1 ml. then MPN is ∠16,000/100 ml.
Environmental Microbiology: Test # 4.
Biochemical Transformation of Carbon Compounds – Isolation of Cellulose Decomposer:
Principle:
ADVERTISEMENTS:
The organic carbon compounds that eventually are deposited in the soil due to death and decay of life forms or as organic excretory products released by the living organisms are degraded by microbial activity. The end products, CO2 and H2O, is released into air or soil.
The most abundant organic material in plants is cellulose. It is readily attached by many species of bacteria, fungi and soil actinomycetes. The steps of cellulose degradation by microbes are as follows:
Cellulose ” Cellobiose ” Glucose ” CO2 + H2O
In general, cellulolytic microorganisms are common in cultivated and forest soils, in manure and in decaying plant tissues. These cellulose decomposers can be isolated in a medium which is supplemented with cellulose.
Requirements:
1. Cellulose agar medium – (K2HPO4 – 1 g/lit., CaCl2 – 0.1 g/lit., MgSO4; 7H2O – 0.2 g/lit., NaCl – 0.1 g/lit., FeCl3 – 0.02 g/lit., NaNO3 – 2.0 g/lit., Agar – 15 g/lit., Precipitation cellulose – 4 g/lit., Distilled water – 1 lit.)
2. Conical flask, Petridis-lies, Culture tubes etc.
3. Garden soil, Incubator etc.
4. Sterilised water.
Procedure:
A. Preparation of Precipitated Cellulose: 100 ml. of conc. sulphuric acid is slowly added to a litre flask containing 60 ml of distilled water. Then the flask is cooled rapidly. Now 1 gm. of moist filter paper chips is added to the acid mixture. Then the content is transferred to a 10 lit. Container filled with distilled water, shaken vigorously and allowed to settle for overnight.
The precipitated cellulose will settle down. The precipitate is recovered and washed thrice with water. During preparation of cellulose agar medium all ingredients are mixed in the appropriate proportion and then sterilised except cellulose precipate, which will be sterilised with water separately and then added to the main part aseptically.
B. Isolation of Cellulose decomposers: 1 gm. garden soil is added to 9 ml. sterilised water and shaken thoroughly. Then a serial dilution up to 10-2 or 10-3 is made. Finally 0.1 ml. of diluted sample is plated on sterilised petridish and then 15 ml. melted medium at 45°C is added and mixed thoroughly. Plate’s arc then cooled and incubated at 28°C for 3 – 5 days.
Observation:
After the period of incubation is over, colonies will appear which arc then purified on repeated sub culturing on cellulose agar medium. In this way the cellulolytic organisms are isolated from the soil.