ADVERTISEMENTS:
Let us make an in-depth study of the methods and basic principles of sterilisation and disinfection. Sterilisation can be effected by the following methods: I. Physical Methods and II. Chemical Methods.
Sterilisation and Disinfection:
These two terms are used to describe the killing or removal of micro-organisms. The sterilisation is an absolute term which denotes the complete killing or removal of microorganisms of all kinds, while the disinfection is a relative term indicating a mere removal of burden of pathogenic microorganisms on the article. Sterilisation is the process of freeing of an article from all living organisms, including viruses, bacteria and their spores, fungi and their spores, both pathogens and non-pathogens. Sterility is an absolute state. An article can never be “relatively sterile”.
Sterilisation of culture media, containers and instruments is essential in bacteriological work for the isolation and maintenance of pure culture. In nursing practice, surgery and medicines, the sterilisation of the instruments, drugs and other supplies is important for the prevention of the disease and it is also required for medical and surgical instruments and materials used in procedures that involve the penetration into the blood, tissues and other normally sterile parts of the body, e.g., surgical operation, intravenous transfusion, hypodermic injections and diagnostic aspirations.
ADVERTISEMENTS:
Disinfection is a method of freeing an article or instrument from some or all living pathogens which may produce the infection during the use of the instruments contaminated with the pathogens. This term is relative since the effectiveness of disinfection depends upon the proportion of the pathogenic microorganisms, killed or removed.
In circumstances where the sterility is not necessary or sterilising procedures are unwanted and impracticable, the disinfection should be adopted, e.g., bedpans, baths, wash basins, furniture, eating utensils, bed clothes, fomites, which may spread the infection in the hospitals, cannot be sterilised but they can be easily disinfected.
Similarly, it is not practicable to apply sterilising procedures to the skin. The non-spore forming bacteria present in the skin mostly infect the surgical wound, hence it is of great necessity to disinfect the operative site as a valuable pre-operative precaution to kill the vegetative bacteria.
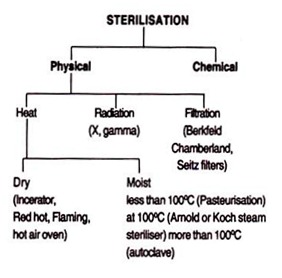
Sterilisation can be effected by the following methods:
ADVERTISEMENTS:
I. Physical Methods:
(a) Heat:
(i) Dry heat (inceration, red hot, flaming, hot air oven, infrared radiation);
(ii) Moist heat (Pasteurization, boiling water, steam sterilizer, autoclave).
(b) Radiation:
(i) Non-ionising radiation (Ultraviolet radiation);
(ii) Ionising radiation (X-ray, gamma ray).
(c) Filtration (Berkfeld, Chamber land, Seitz, sintered glass, cellulose membrane filters).
II. Chemical Methods:
Basic Principles of Sterilisation by Heat:
Moist heat is more effective than dry heat by sterilising at a lower temperature in a given time, or in a shorter time at a given temperature. Moist heat kills the microorganisms by coagulating and denaturating their enzymes and their structural proteins, a process in which the water participates.
Sterilisation, e.g., killing of the most resistant spores requires exposure to moist heat at 121°C for 10-30 minutes. Dry heat is believed to kill the microorganisms by causing destructive oxidation of essential cell constituents. Killing of the most resistant spores by dry heat requires a temperature of about 160°C for 60 minutes in hot air oven.
Factors Influencing Sterilisation by Heat:
1. The temperature and the time of exposure;
2. The number of microorganisms and spores;
ADVERTISEMENTS:
3. The species, strains and spore forming ability.
Chemical:
1. The temperature and the time of exposure for sterilisation are inversely related. At higher temperature, shorter time exposure is sufficient, e.g., the heating must be hot enough. For surgical and bacteriological sterilisation, an exposure of the organisms to moist heat at 121°C for 10-20 minutes is sufficient for killing of all pathogenic spore forming organisms and all saprophytes except some strict thermophiles which grow best at more than 40°C. Sterilising time (holding period) is the time during which the microbes themselves should be held at the given temperature and does not include the heating up time. The amount of time allowed for heating up is known as heat penetration time.
2. The rapidity of the sterilisation is affected by the number of microbes and their spores. In practice, before applying heat for sterilisation, the cleaning of all the articles or instruments should be done to reduce the burden of contaminated bacteria for better and rapid sterilisation.
ADVERTISEMENTS:
3. The susceptibility to heat is affected by the species, strains and spore forming ability of the microorganisms. The amount of heat required to kill a given strain or species of bacteria is related to the temperature and time of exposure and it is stated that the thermal death point when the lowest temperature is required to kill completely the bacteria within 10 minutes or the thermal death time when the shortest time is required to kill completely the microbes at a particular temperature.
The most reliable method of measurement of the thermal death point and time is the decimal reduction time or D value which is the time (in minutes) required to achieve a tenfold reduction in the viability of a bacterial suspension at a given temperature under standard condition.
Effect of Moist Heat:
A temperature of 50°C to 65°C may kill within 10 minutes the vegetative forms of most bacteria, yeasts and fungi and animal viruses. Treponema pallidum is fragile and killed at 43°C in 10 minutes Coxiella burnetii causing Q fever in man is heat resistant. Bacillus stear other mophilus is extremely resistant to heat.
Poliomyelitis virus is killed at 60°C for 30 minutes and serum hepatitis virus is killed at 60°C for 10 hours. Bacteriophages are killed at the temperature of 65-80°C for 15-30 minutes. The most resistant forms of spores of actinomycetes, yeasts and fungi are killed at 80-90°C in 30 minutes. The spores of Clostridium tetani, CI. Welchii are killed at 100°C for 10 minutes, whereas CI. botulinum in 8 hours.
Effect of Dry Heat:
ADVERTISEMENTS:
Dry heat at 160°C for 60 minutes will kill vegetative bacteria and spores which are susceptible to moist heat at 60°C in 30 minutes and heat resistant spores. High content of organic substances protect spores and vegetative forms against the lethal action of heat. The heat resistance of spores is diminished with increased acidity and alkalinity. The effect of alkali is used in the disinfection of metal instruments by boiling at 100°C in water containing 2 per cent sodium carbonate; but this method is not reliable as autoclaving.
Sterilisation by Moist Heat:
Microorganisms can be killed by moist heat if they come in contact with water or steam; otherwise if they are protected from wetting, as by grease or in a relatively impervious container exposed at the same temperature; the moist heat will have the weaker effect than the dry heat.
Method of Sterilisation by Moist Heat:
In sterilisation, moist heat can be used:
(a) At temperature below 100°C;
(b) At a temperature of 100°C (either in boiling water or in free steam); or
(c) At a temperature above 100°C (in saturated steam under increased pressure) which ensures the complete sterilisation and killing of the most highly resistant spores.
Basic Principles of Moist Heat Sterilisation:
ADVERTISEMENTS:
Because of its greatest lethal effect on microorganisms, quicker heating up effect on the exposed articles and its penetrating power through porous materials (paper, cloth wrapper, bundles of surgical instruments, wool stoppers, and cotton), saturated steam is a more efficient sterilising agent than the hot air.
When the cooler surface of the article comes in contact with the steam, the steam condenses into a small volume of water and liberates its large latent heat to that cooler surface of the article (e.g., 1,600 ml of steam at 100°C condense, at atmospheric pressure, into 1 ml of water at 100°C liberating 518 calories of heat).
The contraction in volume causes immediate suction of more steam to the same site and this process continues till the temperature of the article is raised to the temperature of the steam. The condensed water provides moist conditions for killing of the exposed microbes.
Since the penetration by the steam is hindered by the air, the air should not be trapped inside the autoclave; only pure steam is used. The loss of water by evaporation by heating is prevented by the saturated steam. Hence, culture media (both solid and liquid) can be sterilised by the steam under pressure.
In the autoclave or steamer, the cotton wool stoppers can be drenched, this drenching can be avoided by covering the stopper with craft paper. If a wire basket containing test tubes is used, it can be covered with a sheet of paper folded down the edges of the basket.
Moist Heat at a Temperature below 100°C:
The process which kills all non-spore forming pathogens (Mycobacterium tuberculosis, Brucella abortus, Salmonella) in milk by subjecting the milk to a temperature of 63°C for 30 minutes (the holder method) or 73°C for 20 seconds (the flash method) is known as “Pasteurization of milk.”
ADVERTISEMENTS:
Serum or body fluids containing coagulable protein can be sterilised by heating for 1 hour at 56°C on several successive days in water bath. Care must be taken not to allow the temperature to rise above 59°C, which may cause inspissation. Vaccines can be prepared in a vaccine bath at a temperature of 60°C for 1 hour.
Moist Heat at a Temperature of 100°C:
Boiling at 100°C. Boiling at 100°C for 5-10 minutes does not ensure the complete sterility, but it is enough to kill all non-sporing organisms and few spore-forming bacilli (e.g., strains of CI. tetani may survive boiling for 1 -3 hours). Sometimes, in microbiology and medicine, this boiling method is used, when the absolute sterility is unwanted for pipettes, measuring cylinders, rubber stoppers, instruments (scalpels, forceps, scissors, metal or glass syringe) which do not withstand higher temperature.
If water supply is hard, the instruments after removal from boiling water may become covered with a film of calcium salts; to avoid this effect, distilled water should be used. Addition of 2 per cent sodium bicarbonate may promote the sterilisation. Long handed-forceps, kept immersed in 3 per cent Lysol to a level approaching the finger grip, can be used to remove the articles and instruments from boiling water. If the instrument was taken into the hand, while it is wet, its working end (e.g., scalpel blade or syringe needle) may get contaminated with skin bacterium flowing from the fingers in the film of water.
However, boiling water can generally be used for contaminated dishes, bedding and bedpans, because these articles do require neither sterility nor the destruction of spores. Only disinfection is necessary for these articles. At altitudes over 5,000 feet, the boiling time should be increased by 50 per cent, because the water boils at this altitude at a temperature of 95°C or below.
Boiling (at 100°C) for 10 minutes kills the vegetative and actively multiplying cells of pathogenic bacteria, rickettsiae, all viruses except hepatitis and poliomyelitis viruses, if these microorganisms are not protected by the blood, food, faeces, mucus etc. It also kills or damages the cysts of protozoa, ova of helminths, ascospores of pathogenic yeasts, conidia of molds.
Since the spores of Clostridium tetani, Cl.welchii and Bacillus anthracis and hepatitis virus are resistant to boiling even for 10 minutes, so boiling at 100°C for 10 minutes is not effective. Spores present in soil, faeces, dust, on instruments, bandages contaminated with blood, dust may 6e a risk of infection. Where as anthrax spores occur only in areas where the infected animals died of anthrax.
Boiling at 100°C for 10 minutes is a sure means of disinfection (not sterility), if the spores and viruses are not present, of instruments and glassware’s, if they are not soiled with blood, faeces, food etc. The margin of safety can be increased by boiling for 30 minutes.
Summary:
1. Vegetative forms of all bacteria and fungi pathogenic to man; rickettsiae pathogenic to man; helminths and their ova; many viruses (except hepatitis virus); bacterial toxin (except staphylococcal enterotoxin) are killed or inactivated by boiling at 100°C for 10 minutes.
2. Bacterial spores; Hepatitis virus; ascospores of fungi; conidia of some fungi pathogenic to man; staphylococcal enterotoxin are not killed or inactivated by boiling at 100°C for 10 minutes. Steaming at 100°C is commonly used in the microbiological laboratory to sterilize the culture media (both nutrient broth and nutrient agar).
It is not as effective as autoclaving. Koch or Arnold steam sterilizer or “Steamer” is a vertical metal cylinder with a removable lid having a small outlet for the steam escape and at its bottom it contains water which is heated up. Above the water level, a tray bears the articles to be sterilised. Frequent opening of the steamer should be minimised, because the air permitted inside the steamer ultimately interferes with the sterilisation.
Sterilisation can be carried out as follows:
(a) By a single exposure at 100°C for 90 minutes.
(b) By intermittent exposure at 100°C for 20- 45 minutes on each three successive days.
(a) Single exposure at 100°C for 90 minutes is not suitable as the spores of thermophilic and some mesophilic bacteria can survive this exposure.
(b) Intermittent exposure at 100°C for 20-45 minutes on each three successive days is also known as “Tyndallisation” In this method one exposure will destroy all the vegetative organisms between the heating’s, the spores in favourable nutrient medium become vegetative forms which are killed during the subsequent heating. This method is suitable for the medium containing sugars which maybe decomposed at higher temperature and for gelating medium which, after prolonged heating, may not solidify on cooling.
Moist heat at temperature above 100°C:
Sterilisation in the Autoclave:
Principle employed in the autoclave — water boils when its steam pressure equals to atmospheric pressure, or it can be interpreted as the water boils at 100°C at normal atmospheric pressure (i.e., 760 mm Hg; 14 lb. per square inch pressure). So when the water is boiled within a closed vessel at increased pressure, the temperature at which the water boils and that of its steam will rise above 100°C.The most effective method of sterilisation of culture media (in the bacteriological laboratory) and surgical materials or supplies (in the hospital) is Autoclaving.
The steam should perforate all parts of the articles to be sterilised in the autoclave and should be hot, saturated (i.e., point of condensing to liquid water) and dry (i.e., free from particles of liquid water). This dry saturated steam affects the sterilisation because of its high temperature (latent heat).The ideal minimum holding time is 15-30 minutes at 121°C (15 lb. per square inch gauze pressure).
Air Discharge:
To expose all articles in the autoclave to the pure steam during sterilisation, it is necessary to remove all the air from the autoclave, because:
(a) The mixture of steam and air results in a lower temperature at a particular pressure,
(b) The penetration of steam in the interstices of porous materials (e.g., surgical dressings), syringes, etc., may be interfered by the air, and
(c) The air, being denser than the steam from a cooler layer at the bottom of the autoclave, which prevents the sufficient heating of the articles at the lower level.
There are two types of autoclaves:
(a) The simple non-jacketed laboratory autoclave;
(b) Steam-jacketed autoclave with automatic air and condensate discharge.
(a) The simple non-jacketed laboratory autoclave (Fig. 15.1) is also called as “pressure cooker type” It is made up of a vertical or horizontal gun metal or stainless steel cylinder of about 18 inches in diameter and 30 inches in length with a supporting stand. The lid (door) is tightened by screw clamps and is rendered airtight by means of an asbestos washer.
The water level inside the cylinder should be up to 3. 1/2 inches for a vertical cylinder of 19 inches internal length. The autoclave is heated by a gas burner or electric heater from below the cylinder. The articles to be sterilised are placed on a perforated tray situated above the water level. The lid of the autoclave is provided with a discharge tap for the air and steam, a pressure gauze and a safety valve which is adjusted to blow off automatically at any desired pressure.
Operating of the Simple Autoclave:
Inside the autoclave, the water should be up to required level, i.e., below the perforated tray. The materials to be sterilised are placed on the tray and the heater should be turned on. The lid should be placed in position and fastened by screw clamps, the discharge tap should remain open and the safety valve is to be adjusted to the required pressure.
In some autoclave, the pressure has been predetermined and adjusted by the manufacturers. When water boils inside the cylinder of the autoclave, the steam rises, mixes with the air in the chamber and expels out this air through the discharge tap.
The operator should allow the steam and air mixture to escape freely till all the air is completely removed from the autoclave, which can be adjusted by connecting a rubber tube from the discharge tap into a container of cold water into which the steam condenses and the air rises as bubbles to the surface.
This procedure can be followed by an inexperienced operator for his own satisfaction, when there is no trained nurse. If the air has completely escaped, the discharge tap should be closed. The steam pressure rises till it reaches the desired level, e.g., 15 lb. per square inch for 121 °C, when the safety valve opens automatically and allows the excess steam to escape.
From this point, the holding time begins and continues for 15 minutes. The heater should be turned off, the discharge tap should be opened gradually and the autoclave should be allowed to cool till the inside pressure equals to the atmospheric pressure (0 lb. per square inch) which may take about 1 hour; when the chamber pressure is still high, if the discharge tap is opened, there is possibility of danger of explosion of autoclave lid, culture media will boil and spill from the container.
An excess amount of water will be evaporated and lost from the media if the discharge tap is not opened until the pressure has fallen much below the atmospheric pressure. It is necessary to have surgical linen and dressings wrapped in paper or cloth for quick drying of the materials after sterilisation.
These wrapped articles are moistened by the condensation of the steam; when damp, even several layers of paper and cloth wrappings could not check the entry of contaminating bacteria into the sterilised articles. Therefore, the sterilised articles should not be kept in contact with unsterilized objects until their wrappings are dry.
(b) Steam-jacketed autoclave (Fig. 15.2) is mostly used in the hospital to sterilize the linen, surgical materials etc. It is a horizontal cylinder. At the front, there is a swing door which is tightened by screw clamps or by a “capstan head” with its radial bolts and it locks automatically while the chamber pressure is raised. This safety door, locked automatically by pressure, can prevent the possibility of a. dangerous explosion, if the operator opens early the autoclave by mistake.
The autoclave consists of:
(1) A steam supply from outside (e.g., an independent boiler),
(2) A steam jacket which heats the sidewalls of the autoclave,
(3) A channel discharging air from the bottom of the chamber)
(4) A thermometer in the discharge channel, indicating the temperature above the lowest and coolest part of the chamber,
(5) Vacuum system to assist drying of the load,
(6) An air intake with a self-sterilising filter for introducing warm sterile air in the chamber,
(7) A cooling system to hasten the cooling of the liquids without violent boiling,
(8) An automatic control system for heating up, holding, cooling and drying.
Steam Supply:
The steam supplied to the autoclave should be dry, i.e., free from excess of water, and saturated, i.e., not superheated.
Wet Steam:
The steam released from the boiler at a long distance may become “wet” and inefficient sterilising agent because of
(a) Cooling and condensation,
(b) Soaking of porous materials which prevents further penetration, and
(c) The particles of water possess no latent heat. Thus, the pressure of the main steam supply should be usually 55 lb. per square inch. The reduction in pressure dries the steam.
Superheated steam is not satisfactory because it abstracts the water from the exposed materials, may cause the less lethal effect and is still more destructive as dry heat. Operating of steam jacketed autoclave. A space between the double sidewalls of the chamber (Jacket) is filled throughout the day with the steam at 121°C introduced from the boiler. Water of condensation from the steam is drained off through the jacket discharge channel which is thermostatically controlled. The operator should ensure that there is no obstruction of this discharge channel.
Loading of the Chamber:
The articles to be sterilised should be arranged loosely so that the steam circulates freely and the air is displaced.
Heating Up and Air Displacement Period:
The swing door should be closed hermetically. The steam from the jacket should enter through a baffle at the back of the chamber. The pressure and temperature should be maintained in the chamber as in the jacket. The cooler and denser air should be displaced by the steam under pressure.
If more steam is introduced under pressure, it displaces the air downwards through the articles to be sterilised and the air is discharged out through the chamber discharge channel situated at the bottom of the chamber. At the same time, the water of condensation on the cool load and the door of the chamber should be drained off through the same channel. As soon as all free air is eliminated, the inflow of pure steam raises the trap’s temperature to 121 °C, it closes automatically and it prevents further escape. The steam replaces the air in about 5-10 minutes.
Holding Period of Sterilisation:
Holding period will start when the thermometer in the discharge channel reaches the temperature of 121°C.The exact duration of the holding period depends upon the nature of the load. A “near to steam” trap is essential as it opens when the temperature falls by 1 °C below that of the pure steam.
Cooling and Drying Period:
The steam supply to the chamber is stopped at the end of the holding period. The steam inside the chamber starts to cool by loss of heat through the door and pressure falls slowly.
High Pre-Vacuum Sterilizer:
High vacuum surgical sterilizer is the only method of sterilisation that can overcome the effects of bad packing or overloading of the sterilizer. Tightly packed load is heated rapidly and uniformly to the sterilising temperature for a shorter time, e.g., 135°C for 3 minutes.
The damage to heat sensitive materials is avoided by greatly shortening the time. The steam, admitted to the chamber, heats the whole load to 135°C within 2-3 minutes. The holding period is maintained for 3 minutes, the temperature reaches 135°C. The load is then dried within a few minutes by exhaustion of the chamber to a high vacuum.
Automatic Control System:
Carries through the whole sterilisation process — heating up, holding, cooling and drying accurately to the fixed time, temperature and pressure. Once the autoclave is started, no further attention is needed till it is cooled. The error due to negligence can be rectified by the control system. The sterilising process can be reported by the monitoring system, if the fixed temperature falls below at any time.
Temperature Record:
Recording thermometer can help the operator to avoid the errors in timing the holding period.
The overall efficiency of the autoclave can be judged by the following two methods:
Chemical indicators and Spore indicators:
(a) Chemical indicators which show a change of colour or shape after exposure to a sterilising temperature, may be placed under the load.
For example:
(i) A sulphur pellet kept in a small glass tube will show a change of shape by melting when exposed to 120°C for a few minutes,
(ii) Brownel’s control tubes contain a red solution which turns green when heated at 115°C for 25 minutes (Type 1); or 15 minutes (type 2) or at 160°C for 60 minutes. These tubes should be stored at less than 20°C to avoid the deterioration and premature change in colour,
(iii) Bowie- Dick tape, applied to packs and articles in the autoclave, develops diagonal lines when exposed to the sterilising temperature for the correct time,
(iv) Sterilometer is a cellulose tape having on it a chemical indicator which changes its colour when properly heated in the autoclave.
(b) Spore Indicators: Bacillus stearothermophilusgrovjs best at 55°C-60°C. But a preparation of its dried spores is killed at 121°C in 12 minutes when placed within the load in the autoclave. After autoclaving, it can be tested for its viability on the culture media.
Nurse using the autoclave should be sure that:
(a) All the air is allowed to escape and is replaced by the steam;
(b) The pressure of the steam reaches at least 15 pounds to the square inch and remains there;
(c) The thermometer reaches at least 121°C for 20 minutes.
Sterilisation by Radiation:
Nonionizing Ultraviolet Radiation:
Ultraviolet rays of sunlight have the bactericidal effects which depend upon the wave length. It is bactericidal when it reaches a wave length of 330 mn (3,300 A° or Angstrom units). Angstrom is unit of measurement of wave length.
Thus, as the wave length decreases, the effectiveness of the ultraviolet light increases as sterilising agent. The shortest ultraviolet rays of sunlight reaching the surface of the earth have a wave length of more than 200 m µ however, most commonly used mercury vapours can emit even more effective radiations of 240-280 mµ.
High intensity ultraviolet radiation is applied with great precautions to protect the skin, to the site of the operation to check the post operative sepsis. Rays from ultraviolet lamps can reduce the bacterial load in the atmospheric dust. Pathogenic microorganisms on the floor and furniture’s in rooms can be destroyed by the daylight passing through the ordinary glass windows.
Ionising Radiation:
In practice, sterilisation by radiation is achieved by the use of high speed cathode rays (electrons), X-rays and short X-rays (gamma rays) from an apparatus (linear accelerator) or gamma rays from an isotope source (cobalt 60).This method is very costly for hospital use; but it is used commercially only for the sterilisation of pre-packed disposable plastic syringes, transfusion sets and catheters which cannot withstand the heat. It is known as cold sterilisation.
Sterilisation by Filtration:
In this process, the fluids (bacterial fluid cultures) can be freed from bacteria by passing through special filters. This method of sterilisation by filtration is specially useful to prepare toxin from bacterial growth and to sterilize the liquids (sugars, serum, antibiotic solutions) which are liable to be damaged by heat. These sterilising filters should be considered as rendering a liquid, bacteria free, but not virus free and are satisfactory for laboratory purposes; but, in clinical practice, such fluid (e.g., glucose, serum) sterilised by Seitz filtration are not safe.
Type of Filters:
For bacteriological work, the following types of filters are used:
1. Earthenware candles, e.g., Berkefeld, Chamberl and filters;
2. Asbestos and asbestos paper disk, e.g., Seitz filter;
3. Sintered glass filters;
4. Cellulose membrane filters.
ADVERTISEMENTS:
1. (a) Berkefeld Filters:
They are made from a fossil diatomaceous earth found in deposits in Germany and other parts of the world and are of three grades of porosity,e.g., V (Veil) the coarsest, W (Wening) the finest, and Normal (N) intermediate. Berkefeld V is often used. A small organism (Serratia marcescens) should pass through this filter.
(b) Chamberland Filters:
They are made of unglazed porcelain of various grades of porosity. The finer grades will pass only certain viruses of extreme minuteness (e.g., Foot and Mouth Disease virus).The most porous grade L1,clarifying filter, allows many microorganisms to pass. The next three L1, L2 and L3 are comparable with the Berkefeld V, N, and W candles, respectively. These filters can also be used to obtain bacterial toxin.
2. Seitz Filters:
Consist of asbestos disk through which the fluid is passed. The disk is inserted into a metal holder which is jointed tightly by screw clamps. The whole filter is wrapped in kraft paper and sterilised in the autoclave. The asbestos disk is discarded after each filtration and is replaced by another new disk for fresh filtration. The disks are available in three grades: clarifying (K), normal, and “special EK.” The normal and “special EK” do not allow the ordinary test bacterium Serratia marcescens to pass through.
3. Sintered Glass Filters:
They are made of finely ground glass attached to the filtering apparatus and sterilised as in case of Seitz filter. After use, they should be washed with running water in reverse direction and cleaned with warm sulphuric acid to which potassium nitrate is added.
4. Cellulose Membrane Filters:
There are two types of cellulose membrane filters:
The gradocol membrane (older type) consists of cellulose nitrate and the modern membrane filter is made of cellulose acetate. The size of many viruses can be determined by these cellulose membrane filter; these filters can retaia Serratin marcescens.
Advantages of cell membrane filters are:
(a) They are much less absorptive and the rate of filtration is much greater;
(b) Bacteria retained on the surface of this filter can be cultivated by placing this filter on culture media.
5. The development of high efficiency particulate air (HEPA) filters or fiberglass filters has made possible to deliver clean air to an enclosure (cubicle or room).This type of air Alteration together with a system of LAMINAR AIR FLOW is now recommended as air disinfection and used very extensively to produce dust and bacteria free air in vaccine sterility testing laboratory and it is expensive.