ADVERTISEMENTS:
TLRs are transmembrane proteins expressed by cells of the innate immune system, which recognize invading microbes and activate signaling pathways that launch immune and inflammatory responses to destroy the invaders. Mammalian TLRs consist of an extracellular portion containing leucine-rich repeats, a transmembrane region and a cytoplasmic tail, called the TIR (Toll-IL-IR (Interleukin-1-Receptor)) homology domain.
Different TLRs serve as receptors for diverse ligands, including bacterial cell wall components, viral double- stranded RNA and small-molecule such as anti-viral or immunomodulatory compounds. Activation of TLRs occurs after binding of a cognate ligand to the extracellular leucine-rich repeats portion of the TLR. In humans, TLR1, 2,4,5 and 6 are outer membrane associated, and respond primarily to bacterial surface associated PAMPs. The second group, TLR3,7,8 and 9 are found on the surface of endosomes, where they respond primarily to nucleic acid based PAMPs from viruses and bacteria. Upon binding with their cognates, TLRs activate two major signaling pathways.
The core pathway utilized by most TLRs leads to activation of the transcription factor NF-κB (Nuclear Factor-κB) and the MAPKs (Mitogen-Activated Protein Kinases) p38 and JNK (c-Jun N-termal Kinase). The second pathway involves TLR3 and TLR4 and leads to the activation of both NF-kB and another transcription factor IRF3 (Interferon Regulatory Factor-3), allowing for an additional set of genes to be induced, including anti-viral genes such as IFN-β (Interferon-Beta) and others. The innate immune response is a complex set of interactions that have evolved to optimize the response to pathogens. While the structure of the TLRs has been highly conserved, the innate immune response for each organism has selectively been driven to protect against the pathogens found in the host’s environment.
ADVERTISEMENTS:
TLRs and Ligands:
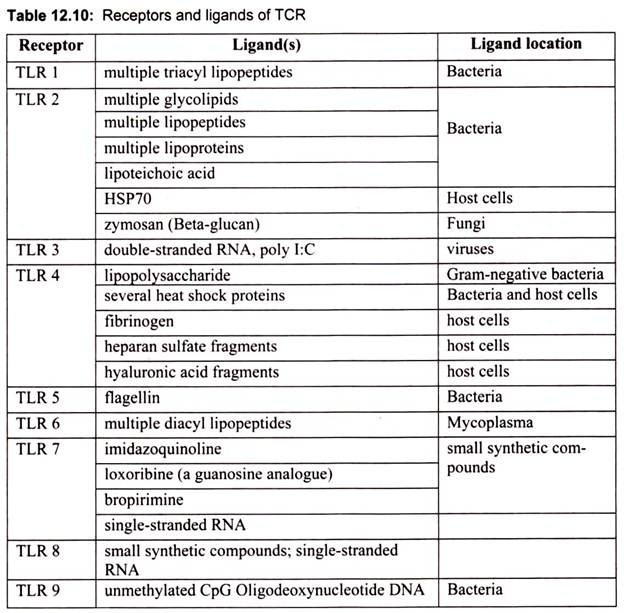
TLRs recognize the specific microbial patterns. Since the last decade there has been a steady increase in the number of TLR family members and their ligands (Table 12.10). Most of the ligand studies are based on the knockout mice. Different TLRs seem to play crucial roles in the activation of the immune response to PAMPs.
TLR1:
TLR1, the first member of the TLR family, was identified by the presence of a domain homology found in both Drosophila Toll and human IL-1 receptors. TLR1 is expressed at higher levels in the spleen and peripheral blood cells. No direct ligands have been identified so far for TLR1, and its function remains unclear. TLR1 seems to act as a co-receptor. TLR1 was shown to associate with TLR2 in response to triacylated lipopeptides, but not diacylated lipopeptides. These observations indicate that TLR1 is able to discriminate among lipoproteins by recognizing the lipid configuration.
ADVERTISEMENTS:
TLR2 and TLR6:
TLR2 has been shown to be involved in the recognition of a broad range of microbial products, including: peptidoglycan from Gram-positive bacteria, bacterial lipoproteins, mycobacterial cell-wall lipoarabinomannan, glycosylphosphatidylinositol lipid from Trypanosoma Cruzi, a phenol-soluble modulin produced by Staphylococcus epidermidis, and yeast cell walls. This unusually broad range of ligands recognized by TLR2 is explained, in part, by cooperation between TLR2 and at least two other TLRs: TLR1 and TLR6. So, the formation of heterodimers between TLR2 and either TLR1 or TLR6 dictates the specificity of ligand recognition.
Thus, TLR2 recognizes a wide range of microbial products through functional cooperation with several proteins that are either structurally related or unrelated. For example, TLR2 cooperates with TLR6 for the recognition of mycoplasmal macrophage-activating lipopeptide 2 kDa (MALP-2). Interestingly, it is TLR6 that discriminates between bacterial lipoproteins, which are triacylated at the amino-terminal cysteine residue, and the diacylated mycoplasmal lipoprotein MALP-2.
TLR 3:
TLR3 recognizes double-stranded RNA (dsRNA), a molecular pattern associated with viral infection. Expression of human TLR3 in the double-stranded RNA (dsRNA)-non-responsive cell line 293 confers enhanced activation of NF-κB in response to dsRNA. In addition, TLR3 -deficient mice are impaired in their response to dsRNA. dsRNA is produced by most viruses during their replication and induces the synthesis of type I interferons (IFN-/α6), which exert anti-viral and immunostimulatory activities. Thus, TLR3 is implicated in the recognition of dsRNA and viruses.
TLR4:
TLR4 is the principal LPS receptor. LPS, a major component of the outer membrane of Gram-negative bacteria is composed of polysaccharides extending outward from the bacterial cell surface and a lipid portion, lipid A, which is embedded in the cell surface. LPS can provoke a variety of immunostimulatory responses; for example, production of proinflammatory cytokines such as IL-12 and inflammatory effector substances such as nitric oxide. Lipid A portion of LPS is mainly responsible for its biological activities. LPS can cause a clinically life-threatening condition called endotoxin shock. In addition to TLR4, a glycosylphosphatidylinositol anchoring protein, CD 14, has been identified that facilitates LPS action by binding and retaining LPS on the cell surface.
TLR5:
ADVERTISEMENTS:
TLR5 recognizes flagellin from both Gram-positive and Gram-negative bacteria. Flagellin is the monomeric subunit of bacterial flagella. Flagellin shows potent pro-inflammatory activity by inducing expression of IL-8. TLR5 was identified by the presence of the TIR domain and is expressed in the spleen, peripheral blood leukocytes and epithelial cells. Enforced expression of human TLR5 in CHO cells confers response to flagellin, a monomeric constituent of bacterial flagella. TLR5 has further been shown to recognize an evolutionarily conserved domain of flagellin through close physical interaction between TLR5 and flagellin. TLR5 is expressed on the basolateral, but not the apical side of intestinal epithelial cells. TLR5 expression is also observed in the intestinal endothelial cells of the subepithelial compartment.
TLR7 and TLR8:
TLR7 and TLR8 are structurally highly conserved proteins, and recognize the same ligand in some cases. Analysis of TLR7-deficient mice revealed that murine TLR7 recognize synthetic compounds, imidazoquinolines, which are clinically used for treatment of genital warts associated with viral infection. Human TLR7 and TLR8, but not murine TLR8, recognizes imidazoquinoline compounds. Murine TLR7 has also been shown to recognize another synthetic compound, loxoribine, which has anti-viral and anti-tumor activities. Both imidazoquinoline and loxoribine are structurally related to guanosine nucleoside.
TLR9:
ADVERTISEMENTS:
TLR9, which is localized intracellularly, is involved in the recognition of specific unmethylated CpG-ODN sequences that distinguishes bacterial DNA from mammalian DNA. CpG oligodeoxynucleotides (or CpG ODN) are short single stranded synthetic DNA molecules that contain a cytosine “C” followed by a guanine “G”. The “p” refers to the phosphodiester backbone of DNA, however some ODN have a modified phosphorothioate (PS) backbone. Bacterial DNA can stimulate immune cells. This activity is mainly because of the unmethylated CpG motifs, which are rarely detected in vertebrate DNA and, if present, are highly methylated. This stimulation leads to the production of Th1 (T helper 1) cytokines and co- stimulatory molecule upregulation.
TLR Activation:
ADVERTISEMENTS:
Unchecked TLR activation by pathogens can lead to serious medical consequences, such as sepsis and autoimmune diseases. In the last few years, negative modulators of TLR activation have been identified, and their important role in reducing the inflammatory response has been demonstrated in animal models. The TAM family members are one example. The TAM (Tyro3/Axl/Mer) family has been found to be central to the fine tuning of the TLR response.
Loss of function of the three members of this family (Tyro3/Axl/Mer) in a triple knockout mouse results in a profound disregulation of the immune response. This includes massive splenomegaly and lymphadenopathy, lymphocyte infiltration into all tissues, and high levels of autoimmunity. Even a single knockout of just Mer is sufficient to elevate susceptibility to LPS induced shock via the TLR4 signaling.
These mice have elevated levels of dendritic cells, and the cells express elevated levels of activation markers, including MHC class II antigens. This effect was not restricted to TLR4, as hypersensitivity to the TLR3 activator polyIC was also observed.
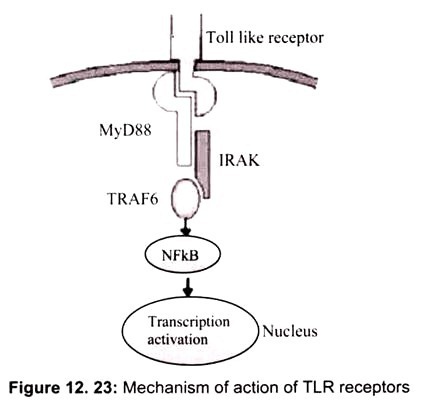
Figure 12.23 shows how TLR receptor works. Recognition of an appropriate ligand (for example, lipopolysaccharide) triggers the Toll-like receptor to recruit MyD88. MyD88 interacts with the Toll-like receptor through its own Toll/IL-1 receptor domain and in turn engages the serine-threonine kinase IRAK though a death domain. Signal transduction factors such as TRAF6 carry the signal through a series of phosphorylations until NFkB is ultimately released to the nucleus where it can activate the transcription of appropriate genes.