ADVERTISEMENTS:
In this article we will discuss about the industrial production of amino acids by fermentation. Learn about:- 1. Industrial Production of Amino Acids 2. Industrial Production of Amino Acids by Fermentation 3. Amino Acid Production Processes 4. Microbial Production of Amino Acids 5. Production of Amino Acids By Fermentation 6. Production of Amino Acids By Microorganisms.
Introduction to Fermentative Production of Amino Acids:
Fermentative production of amino acids has become an industrial reality by the discovery of an efficient glutamic acid producer, Corynebacterium glutamicum (synonym Micrococcus glutamicus), by Kinoshita et al. (1957). The bacterium was found during a time of increasing demand for monosodium glutamate as a flavoring agent, and following this, much research activity has been focused on microbial amino acid production.
The primary reason for these efforts was the hope of improving the nutritional value of low-cost vegetable proteins by enrichment with essential amino acids. Once C. glutamicum was discovered by screening isolates from nature, similar efforts led to the isolation of bacteria producing DL-alanine or L-valine.
ADVERTISEMENTS:
However, it was found that most wild type strains isolated from nature could not produce industrially significant amounts of other amino acids except the few amino acids already referred to. One of the main reasons is that regulation of cellular metabolism avoids over-synthesis. The existence of these regulatory phenomena was just being clarified at the time of the isolation of C. glutamicum and is now well recognized.
An auxotrophic mutant which cannot produce the regulatory effector or corepressor (usually the end product or a derivative of the end product) overproduces and excretes the precursor or the related metabolite of a blocked reaction when grown on a limiting supply of the required nutrient. This is the principle of the application of an auxotrophic mutant to the microbial production of amino acids.
Active attempts to utilize this phenomenon for the industrial production of microbial metabolites were launched in the 1950s and many amino acids are now produced with auxotrophic mutants. It is obviously useless to accumulate the end product of an un-branched pathway such as arginine and histidine with an auxotrophic mutant. The production of such a metabolite depends on the use of a regulatory mutant. A mutant which has lost some biosynthetic regulation can be obtained by selection of an analog-resistant and prototrophic revertant from the auxotroph having a deficiency in a regulatory enzyme.
To improve the yield of an amino acid, mutants having multiple markers, including auxotrophy and analog-resistance contributing to production of the designated amino acid, are selected. Multiple markers also contribute to the yield by stabilizing the productivity against back mutation during fermentation. With metabolic regulation, the permeability barrier is another mechanism which protects microorganisms from leaking organic compounds to the environment. It allows cells to retain intermediates and macromolecules necessary for the life of the microorganisms.
ADVERTISEMENTS:
Permeability is another important factor for amino acid production. In fact, excess production of L-glutamic acid by C. glutamicum was found to be due mainly to the permeability change induced by limiting the supply of biotin required by the bacterium.
Although certain specific environmental conditions make it possible to exploit a single enzymic process or a process using a precursor, most amino acids can now be produced by the so-called “direct fermentation” process, i.e., microbial production from a cheap carbon source by fermentation.
Many processes for production of various amino acids have been developed. Total world production of L-glutamic acid is considered to be in excess of 150,000 MT (165,000 tons) per year. It is used mainly as a flavoring agent. L-lysine has also been produced on a large scale by fermentation and is used mainly as a feed-supplement. Total world production of it is probably in excess of 35,000 MT (38,500 tons) per year.
Methionine, alanine, glycine, and cysteine are not produced commercially by fermentation. The racemic forms of methionine, alanine, and glycine are produced by chemical synthesis, and L-methionine and L-alanine can be made from the racemic form by an enzymatic process. Some other amino acids are produced by fermentation on a scale of less than 1000 MT (1100 tons) per year.
L-Glutamic Acid and L-Glutamine:
Glutamic Acid Production from Carbohydrate:
Production of L-glutamic acid and L-glutamine has been reviewed by Kinoshita and Tanaka (1972). Production of glutamic acid from carbohydrate in high yield is carried out by a group of bacteria represented by Corynebacterium glutamicum (synonym Micrococcus glutamicus). These bacteria are classified as species in different genera – they include Corynebacterium glutamicum (Micrococcus glutamicus), Brevibacterium flavum, B. lactofermentum, B. divaricatum, B. thiogenitalis, Corynebacterium callunae, C. herculis, Microbacterium ammoniaphilum, and others.
The guanine + cytosine (G-C) content of the DNA of these bacteria falls into a narrow range from 51.2 to 54.4 moles %. Morphological and physiological properties also support their close affinities, and they could be classified into a group of bacteria belonging to the Corynebacteriaceae. For convenience these bacteria will be referred to as “glutamic acid bacteria” hereafter.
The special conditions which allow these bacteria to excrete large amounts of glutamate are a nutritional requirement for biotin and the lack, or very low content, of α-ketoglutarate dehydrogenase. The biotin requirement is the major controlling factor in the fermentation. When enough biotin is supplied for optimal growth, the organism produces lactate. Glutamate is excreted under conditions of suboptimal growth.
Under optimal culture conditions, glutamic acid bacteria convert about 50% of the supplied carbohydrate into L-glutamic acid with little formation of by-products. Various carbohydrate materials can be used as the carbon source. Glucose and sucrose are particularly suitable. For industrial purposes, hydrolyzed starch solutions, cane molasses, and beet molasses are preferred.
Other carbon sources such as acetic acid and ethanol are also used. Carbon sources, such as cane molasses, with a high content of biotin are used with the addition of penicillin during logarithmic growth or of fatty-acid derivatives such as polyoxyethylene sorbitan-monooleate (Tween 60) before or during logarithmic growth.
ADVERTISEMENTS:
Ammonium sulfate, ammonium chloride, ammonium phosphate, aqueous ammonia, ammonia gas, and urea have been used as nitrogen sources. Although a large amount of ammonium ion is necessary, a high concentration of it is inhibitory to growth of the organism as well as to production of glutamic acid. Therefore, ammonium ions are added as the fermentation progresses. Ammonia water, or gaseous ammonia, is generally used industrially.
Other ions supplied include K+, Mg2 +, Fe2+, Mn2+, PO43-, SO42- and CI–. These are usually supplied by the following inorganic salts (% w/v) – 0.05-0.2 KH2PO4, 0.05-0.2 K2HPO4, 0.025-0.1 MgSO4.7H2O, 0.0005- 0.01 FeSO4-7H2O, 0.0005-0.005 MnSO4.4H2O and 0.5-4 CaCO3.
The most important factor in the medium for the glutamic acid fermentation is biotin, which is an essential growth factor for glutamic acid bacteria. The concentration of biotin must be suboptimal for growth. The best concentration of biotin for the glutamic acid fermentation depends on the strain, kind, and concentration of the carbon source, but it is generally somewhat below 5 μg per liter of medium. Some strains require thiamin or cystine in addition to biotin.
Certain iron-chelating compounds are necessary for growth of glutamic acid bacteria, but their presence is not necessary when the carbohydrate is autoclaved simultaneously with other ingredients of the medium because iron-chelating compounds are formed by autoclaving.
ADVERTISEMENTS:
The pH value optimal for growth and glutamic acid production is 7.0-8.0. Continuous feeding of NH4+ can adjust the pH value and also supply ammonium ions to the medium. Urea can replace the ammonium ion in those glutamic acid bacteria which possess urease activity. The optimal value of Kd (the overall coefficient of oxygen transfer) for glutamic acid fermentation is considered to be 3-5 x 10-6 (mol O2) atm-1min-1ml-1.
Under conditions of insufficient oxygen, production of glutamic acid is poor and large amounts of lactic acid and succinic acid accumulate, while excess oxygen increases the amount of lactic acid and α-ketoglutaric acid. The optimal temperature for the glutamic acid fermentation is usually 30° to 35°C.
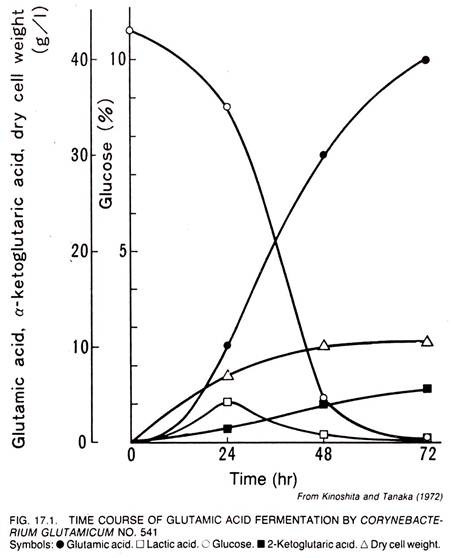
The time course of the glutamic acid fermentation by C. glutamicum No. 541 is shown in Figure 17.1. The medium used had the following composition (w/v) – 10% glucose, 0.05% K2HPO4, 0.05% KH2PO4, 0.025% MgSO4.7H2O, 0.001% FeSO4.7H2O, 0.001% MnSO4.4H2O, 0.5% urea, and 2.51μg/liter biotin. The fermentation was carried out in 5 liter jar fermentors at 28°C. The pH value of the medium was kept between 7 and 8 by feeding of Urea.
Glutamic Acid Production from Non-Carbohydrate Materials:
ADVERTISEMENTS:
The availability of acetic acid at a reasonable price and the waste water problem associated with the use of cane molasses as the carbon source for the glutamic acid fermentation prompted the search for a process using acetic acid. With Brevibacterium flavum, L-glutamic acid production reached 98 g per liter (48% on the basis of acetic acid) in 48 hr.
Adding 25 to 250 μg of Cu2+ per liter to a medium increased L- glutamate yield from acetate by B. thiogenitalis. The poor yield of L- glutamate from acetate by copper-deficient cells seems to be due to a decrease in energy supply which is caused by the low efficiency of oxidative phosphorylation. The productivity of an oleic acid auxotroph of B. thiogenitalis was superior to the parent strain. Brevibacterium sp. B 136 converted ethanol to L-glutamic acid with a yield of 60%.
A group of bacteria represented by Nocardia erythropolis (synonym Corynebacterium hydrocarboclastus) produces L-glutamic acid from n-paraffins in media containing suboptimal concentrations of thiamin. The glutamic acid yield is stimulated by addition of penicillin to an exponentially growing culture with excess thiamin in the medium.
ADVERTISEMENTS:
Cupric ions stimulate growth and glutamic acid production from n-paraffins by Arthrobacter paraffineus. Production of glutamic acid by A. paraffineus reached 82 g per liter at 48 hr. A penicillin-resistant mutant of N. erythropolis produced 84 g of L-glutamic acid per liter. A glycerol auxotroph of C. alkanolyticum produced 72 g of L-glutamic acid per liter.
Biosynthetic Pathway from Glucose:
The major route, shown with the heavy arrows, involves at least 16 enzymic steps. α-Ketoglutarate is converted to glutamate by reductive amination. The enzyme catalyzing this conversion is the NADP-specific glutamate dehydrogenase. The presence of this enzyme is essential for glutamate formation.
When resting cells are incubated with glucose in the absence of ammonia, α-ketoglutarate accumulates rather than glutamate. The NADPH required for the action of glutamate dehydrogenase is supplied by the preceding isocitrate dehydrogenase reaction. Glutamate dehydrogenase, in turn, provides the NADP required for isocitrate dehydrogenase activity.
A low content of α-ketoglutarate dehydrogenase favors glutamate production. The importance of the lack of this enzyme has been demonstrated with E. coli, which does not require biotin and is not a glutamate excreter. Even without the biotin requirement, a mutant which lacked α-ketoglutarate dehydrogenase was found to excrete 2.3 g of glutamate per liter while its parent excreted none.
In addition to the EMP pathway, glutamic acid bacteria also use the HMP pathway to convert glucose to 3-carbon and 2-carbon compounds. These compounds can then feed into the TCA cycle. Estimates of the relative utilization of glucose by the two pathways have shown the predominance of the EMP pathway under fermentation conditions.
When glutamate fermentation is carried out in the presence of 14CO2, the radioactivity is fixed into the a-carboxyl group of glutamate. Two enzymes have been found in glutamate excreters which participate in the fixation of carbon dioxide, namely, oxalacetate carboxylase and the NADP-linked malic enzyme which catalyzes fixation of carbon dioxide to pyruvate to yield malate.
ADVERTISEMENTS:
Malate is oxidized to oxaloacetate by malate dehydrogenase, and then oxaloacetate is converted to citrate. Therefore, the two competing reactions of isocitrate are important. During the growth phase, the isocitritase reaction is needed for energy production and to produce intermediates for biosynthetic reactions.
However, after the growth phase, glutamate production would be better without operation of the isocitritase reaction. This would indicate that optimal conditions for the growth phase and for the glutamate production phase should be different.
After growth, the ideal fermentation would proceed by the following reaction:
This represents a 100% molar conversion or 81.7% weight conversion of sugar to glutamic acid. On the other hand, the poorest fermentation would be represented by complete oxidation of glucose as occurs during growth in media containing an optimal concentration of biotin and resulting in no conversion.
What is actually found lies between these limits, i.e., 50-75% molar conversion by resting cells. This indicates that carbon is being lost as carbon dioxide by reversal of action of the malic enzyme and of oxaloacetate carboxylase during operation of the glyoxylate bypass. Therefore, assimilation of ammonium ions in this organism is almost totally dependent upon action of NADP-linked glutamate dehydrogenase.
Alteration of Permeability Relevant to Glutamic Acid Production:
ADVERTISEMENTS:
Alteration of permeability in relation to glutamic acid production was reviewed by Demain and Birnbaum (1968). Production of a large amount of glutamic acid in bacteria is attained by the alteration of the permeability barrier. Increased permeability can be induced in glutamic acid bacteria by either biotin deficiency, oleic acid deficiency in an oleic acid auxotroph, glycerol deficiency in a glycerol auxotroph, treatment with fatty acid derivatives, or by addition of penicillin, cephalosporin C, or T-125 (tunicamycin-like antibiotic) to the growth medium. Biotin deficiency and treatment with fatty acid derivatives cause an aberration in the normal synthesis and distribution of cellular fatty acids.
The cell membrane from such cells contains an abnormal ratio of saturated to unsaturated fatty acids. This abnormality directly correlates with increased permeability of the cell and with the excretion of high concentrations of glutamic acid.
Biotin auxotrophs and oleic acid auxotrophs cannot be used for the production of L-glutamic acid from n-paraffins. Excretion of L-glutamic acid by addition of penicillin or cephalosporin was accompanied by excretion of both phospholipid and N-acetylglucosamine, which are known to be components of the cell membrane and the cell wall, respectively.
Based on this observation, a glycerol auxotroph of Corynebacterium alkanolyticum was isolated. In the auxotroph, cellular phospholipid synthesis was regulated by the amount of glycerol supplied. The mutant produced about 40 g of L-glutamic acid per liter from n-paraffins in the culture in the presence of 0.01% of glycerol but in the absence of penicillin. Studies with the auxotroph suggest that the permeability of L- glutamic acid is not always controlled by the cellular content of unsaturated fatty acyl residues and that phospholipid content controls the membrane permeability of L-glutamic acid.
Addition of penicillin to log phase cultures of C. glutamicum results in a rapid 97-99.5% decrease in viability accompanied by a rapid rate of glutamate excretion. Cell mass represented by optical density and total cell count remain fairly constant after a slight initial increase. The packed cell volume, however, decreases by 60-80% during the phase of decreasing viability indicating a change in the surface properties of the cells.
The cells continue to produce glutamate for 40-50 hr after penicillin addition with no lysis throughout the entire fermentation. Apparently, the lack of an overabundance of potent mucopeptidases allows the cells to retain their form and to metabolize as permeable “resting” entities.
During growth in a medium containing a high concentration of biotin, C. glutamicum synthesizes glutamate until the cell becomes saturated at 25-35 μg per mg of dry weight. Cessation of production is assumed to be due to some feedback control of glutamate toward its own synthesis. When the cells become more permeable, glutamate passes from the cells into the medium, thus releasing the feedback effect and allowing further synthesis.
L-Glutamine and N-Acetyl-L-glutamine:
L-Glutamine and N-acetyl-L-glutamine are produced along with glutamic acid under certain conditions by glutamic acid bacteria. Production of glutamine is increased by maintaining the medium at a weakly acidic pH value, and production of N-acetylglutamine is increased by maintaining a neutral to weakly acidic pH value in the medium.
Under optimal conditions, a 20% conversion of glucose to glutamine and over 20% to N- acetylglutamine are observed. High concentrations of ammonium ion retard growth and glutamic acid production. Increase in the biotin supply and addition of natural nutrients such as corn-steep liquor and meat extract to the medium stimulate growth and increase glutamine production.
These nutrients or Zn++ at a concentration over that required for growth represses formation of N-acetylglutamine. Selection of a suitable strain (KY 9003) and limiting the Zn++ concentration in the medium permitted preferential N-acetylglutamine production. L-Glutamine and N-acetylglutamine are used in the treatment of gastric ulcers.
L-Lysine:
L-Lysine Production by Fermentation:
Microbial production of L-lysine was reviewed by Nakayama (1972B). A microbial process for L-lysine production was first developed by a combination of diaminopimelate production by a lysine auxotroph of Escherichia coli and decarboxylation of the compound by Aerobacter aerogenes or wild type E. coli. Direct production of L-lysine from carbohydrate was developed first with a homoserine (or threonine plus methionine)-auxotroph of Corynebacterium glutamicum. The same type of process was reported with a homoserine auxotroph of Brevibacterium flavum. The leaky homoserine auxotroph was recognized as a threonine-sensitive mutant because growth was inhibited by excess threonine and the inhibition was released by addition of methionine.
This phenomenon is due to feedback inhibition of residual homoserine dehydrogenase by threonine. Homoserine (or threonine plus methionine) auxotrophs of other bacteria were also found to produce L-lysine, but the yields were lower than that from the homoserine auxotroph of coryneform bacteria.
Threonine auxotrophs and leucine auxotrophs of C. glutamicum produce fairly large amounts of L-lysine, but they are inferior to the homoserine auxotroph. Other auxotrophs of C. glutamicum and other bacteria were also inferior to the homoserine auxotroph of C. glutamicum.
Double auxotrophs, which require, in addition to homoserine, at least one of the amino acids, threonine, isoleucine, or methionine, for growth, have been found to be highly stabilized, showing little tendency to revert to homoserine independence. It is possible not only to prevent reversion of the cultures to a wild type state, but also to produce lysine in higher yields since many of the microorganisms are double mutants in the homoserine pathway.
Cane molasses is now generally used as a carbon source in the industrial production of lysine, although other carbohydrate materials, acetic acid, and ethanol can be used. The pH value of the medium is maintained near neutrality during the fermentation by feeding ammonia or urea. Ammonium salts are generally good nitrogen sources, and urea can be used for organisms having urease activity.
An example of a fermentation using cane molasses in a 2 kl fermentor is as follows. The medium for first seed culture contained 2% glucose, 1% peptone, 0.5% meat extract, and 0.25% NaCl in tap water. For the second seed culture, the medium contained 5% cane molasses, 2% (NH4)2SO4, 5% corn-steep liquor, and 1% CaCO3 in tap water.
For the fermentation, the medium contained 20% reducing sugars expressed as invert (as cane molasses) and 1.8% soybean meal hydrolysate (as weight of meal before hydrolysis with 6 N H2SO4 and neutralization with ammonia water) in tap water. The fermentation was carried out at 28°C.
Figure 17.3 shows the time course of the fermentation using C. glutamicum No. 901 (a homoserine auxotroph), which produced 44 g of L-lysine per liter in 60 hr. Foaming in the aerated culture can be repressed by addition of proper antifoaming agents. The amount of the growth factors (homoserine or threonine and methionine) should be appropriate for the production of L-lysine.
It is supplied in limited amounts and is suboptimal for growth. The biotin concentration in the medium must generally be greater than 30 μg per liter. With a limited supply of biotin, L-glutamate accumulates in place of L-lysine. Cane molasses usually supplies enough biotin. Yields of L-lysine as the monohydrochloride reach 30-40% in relation to the initial sugar concentration.
Coryneform glutamic acid-producing bacteria can utilize acetic acid as a carbon source for growth and lysine production. L-Lysine production from acetic acid by a homoserine-leaky (threonine-sensitive) threonine auxotroph mutant of Brevibacterium flavum reached 75 g (as monohydrochloride) per liter or 29% on the basis of acetic acid and glucose supplied.
The medium contained 0.7% acetic acid, 0.2% KH2PO4, 0.04% MgSO4.7H2O, 0.001% FeSO4.7H2O, 0.001% MnSO4.H2O, 3.5% hydrolysate of soybean protein, 3.0% glucose, 50 μg biotin per liter, and 40 μg thiamin-HCl per liter (pH 6.0). Fermentation was carried out at 33°C with feeding of a solution of acetic acid.
The feeding solution contained 60% acetic acid composed of a mixture of acetic acid and ammonium acetate having a molar ratio of 100:25, and 3% glucose. Feeding was controlled automatically until the end of the fermentation, keeping the pH value of the medium at 7.4.
A mutant of Brevibacterium flavum resistant to 5-β-aminoethyl L-cysteine (AEC), a lysine analog, produced fairly large amounts of L-lysine. The increase in lysine yield (more than 10%) was obtained using a mutant of C. glutamicum, which requires homoserine and leucine and is resistant to AEC.
It produced 39.5 g of L-lysine per liter in a medium containing 10% reducing sugars expressed as invert (as cane molasses) while the homoserine plus leucine auxotroph produced 34.5 g of L-lysine per liter. Some patents have been issued for the process to produce L-lysine from n-paraffin.
A similar regulatory pattern was also observed in B. flavum. The blocking of homoserine synthesis at homoserine dehydrogenase results in the release of the concerted feedback inhibition by threonine and lysine on aspartokinase, and the aspartic semialdehyde produced proceeds to lysine through the lysine synthetic pathway on which no feedback inhibition is found, a situation which differs from that in E.coli.
Resistance to AEC brought about by the desensitization of aspartokinase also releases the concerted feedback inhibition. The conversion of aspartic semi-aldehyde to threonine is feed- back-inhibited by L-threonine. Thus the overproduced aspartic semi-aldehyde is channelled into L-lysine production.
L-Lysine Production from DL-α-Aminocaprolactam:
L-lysine production from DL-α-aminocaprolactam was first found with Aspergillus ustus. However, the yield was low. More recently, a very efficient process has been developed for the conversion. Incubation of a mixture of 100 ml of 10% DL-α-aminocaprolactam (adjusted to pH 8.0 with HCl), 0.1 g acetone-dried cells of Cryptococcus laurentii, and 0.1 g acetone-dried cells of Achromobacter obae nov. sp. with gentle shaking at 40°C for 24 hr resulted in the conversion of DL-α-aminocaprolactam to L-lysine in 99.8% yield.
Cryptococcus laurentii produces L-aminocaprolactam hydrolase inductively in a medium containing L- α-aminocaprolactam, glucose, and other ingredients. Achromobacter obae produces aminocaprolactam racemase using both D- and L-α-aminocaprolactam as an inducer. A similar optimal pH value of both enzymes allows the efficient conversion in what appears to be a single step.
L-Threqnine, L-Homoserine, and L-Serine:
L-Threonine:
L-Threonine production from L-homoserine has been studied by several groups, but it has been unsuccessful as an industrial process because of the high cost of homoserine. Direct production of L- threonine from carbohydrate was pioneered by Huang (1961), but its industrial establishment was delayed until Nakayama’s group established the process using an E. coli auxotroph.
Using a diaminopimelate auxotroph and diaminopimelate plus methionine double auxotroph of E. coli, Huang obtained L-threonine production at 2-4 g per liter. Triple auxotrophs of E. coli, which require diaminopimelate, methionine, and isoleucine, and their isoleucine revertants, produced L-threonine in increased yields. One of the isoleucine revertants, KY 8280, produced 13.5 g of L-threonine per liter.
The cultivation was carried out in 5 liter jar fermentors containing 3 liters of a medium having the following composition- 7.5% fructose, 1.4% (NH4)2SO4, 0.3% KH2PO4, 0.3% MgSO4.7H2O,2.0% CaCO3 (pH 7.8). The time course is shown in Fig. 17.5.
The process using the E. coli auxotroph has also been developed by Hirakawa’s group. They isolated a methionine auxotroph (No. 15) from E. coli C-6. It produced 4.3 g of L-threonine per liter in a medium containing 5% reducing sugars expressed as invert (as cane molasses).
A methionine plus valine-leaky double auxotroph (No. 234), derived from strain No. 15, produced 13.6 g of L-threonine per liter in a medium containing 10% glycerol. In strain No. 15, the lysine- or methionine-sensitive aspartokinase, which is insensitive to feedback inhibition, was de-repressed about 5-fold when the auxotroph was cultured in the presence of a limited concentration of methionine.
The production of L-threonine by E. coli auxotrophs was greatly increased by the presence of borrelidin. Addition of aspartic acid with borrelidin further increased the production. The maximum amount of L-threonine accumulated by E. coli No. 234 was 15 g per liter in the medium containing 50 g of glucose and 5 g of sodium aspartate per liter. Borrelidin, an antibiotic, is known to selectively inhibit the activity of the threonyl-tRNA synthetase of some microorganisms.
L-Threonine production by auxotrophic mutants is also encountered in other members of the Enterobacteriaceae and Candida guilliermondii var. membranaefaciens. A mutant deficient in threonine dehydrogenase and threonine dehydratase was isolated from Serratia marcescens. A mutant resistant to α-amino-α-hydroxyvaleric acid (AHV, an analog of L-threonine), derived from the above mutant, produced 14 g of L-threonine per liter.
The regulation of biosynthesis in glutamic acid-producing bacteria as represented by C. glutamicum is different from that in E. coli. Therefore, the organism could not be used successfully for the production of L-threonine. But L-threonine production could be obtained by using a regulatory mutant.
An AHV-resistant mutant of Breuibacterium flavum BB 82 produced 13.5 g of L-threonine per liter in a medium having the following composition- 10% glucose, 3% (NH4)2SO4,1.5% KH2PO4,0.04% MgSO4.7H2O,2 ppm Fe2+, 2 ppm Mn 2+, 200 μg biotin per liter, 300 μg thiamin-HCl per liter, 4 ml Mieki (an HCl-hydrolysate of soybean protein) per liter, 5% CaCO3, pH 7.2 (adjusted with KOH).
Strain BBM-21, a methionine auxotroph derived from another AHV-resistant mutant of B. flavum, produced about 18 g of L-threonine per liter. A methionine auxotroph of C. glutamicum resistant to AHV and thialysine produced 14 g and 10 g, respectively, of L-threonine per liter in a medium containing 10% or 5% reducing sugars expressed as invert (as cane molasses).
Another AHV- and thialysine resistant methionine auxotroph was found to produce both 9 g of L-methionine per liter and 5.5 g of L-lysine. Production of L-threonine with an AHV-resistant mutant was reported with other bacteria including Proteus rettgeri and Corynebacterium acetoacidophilum.
An example of L-threonine production from acetic acid by an AHV- resistant mutant of B. ftavum was reported by Tanaka et al. (1971). The fermentation medium had the following composition- 0.4% ammonium acetate, 0.41% sodium acetate, 1.0% (NH4)2SO4, 0.2% urea, 0.3% KH2PO4, 0.04% MgSO4.7H2O, 0.001% FeSO4.7H2O, 0.001% MnSO4.4H2O, 50 μg biotin per liter, 5 mg thiamin-HCl per liter, 1.5% hydrolysate of soybean protein, and 0.3% glucose (pH 7.2).
After inoculation of the seed culture, the fermentation was conducted at 31°C with automatic maintenance of the pH value at 7 by feeding a mixed solution of acetic acid and ammonium acetate having a molar ratio of 100:17. After 48 hr culture, L-threonine production reached 27 g per liter (14% on the basis of acetic acid and glucose).
An isoleucine-leaky auxotroph of Arthrobacter paraffineus produced L- threonine and L-valine each at 9 g per liter. Besides these amino acids, 2 g per liter each of L-serine and L-leucine were produced. Some of the double auxotrophs derived from the isoleucine auxotroph, and some of their revertants with respect to isoleucine requirement, produced more threonine than the original isoleucine auxotroph. A revertant derived from a methionine plus isoleucine double auxotroph produced 12 g of L-threonine per liter in 7 days incubation with the medium containing 10% n-paraffin (C12-C14 rich). Formation of valine as a byproduct was decreased in this strain.
Corynebacterium glutamicum has one homoserine dehydrogenase which is threonine-sensitive and methionine-repressible, and one aspartokinase which is concerted feedback-inhibited by threonine plus lysine. On the other hand, E. coli has two homoserine dehydrogenases, one of which is methionine-repressible and threonine-insensitive while the other is threonine-sensitive and threonine-repressible.
Furthermore, phosphorylation of aspartate in E. coli is catalyzed by 3 isoenzymes of aspartokinase, one of which is repressible by methionine and insensitive to threonine and is a complex with the homoserine dehydrogenase repressible by methionine; the second one is multivalent, repressible by threonine and isoleucine, and inhibited by threonine, and forms a complex with the homoserine dehydrogenase sensitive to threonine; the third aspartokinase is repressible and inhibited by lysine.
These regulatory processes explain the contribution of methionine, lysine, and isoleucine deficiencies to threonine overproduction in E. coli. Isoleucine auxotrophy contributes also to blocking metabolism of the threonine produced.
The genealogy of the threonine-producing strains of C. glutamicum is shown in Fig. 17.7. The activities of homoserine dehydrogenase in the mutant which produced L-threonine or L-threonine together with L-lysine were slightly less susceptible to inhibition by L-threonine than the activity in the original methionine auxotroph (KY 9159) from which the threonine producers were derived.
The genetic alteration of the enzyme may be one of the causes of L-threonine production in these mutants. The aspartokinase in the threonine-producing mutants (KY 10484 and 10230), which are resistant to AHV and more sensitive to AEC than the parent, were sensitive to concerted feedback inhibition by L-lysine and L-threonine to the same degree as KY 9159.
The aspartokinase activity in KY 10440 was less susceptible to concerted feedback inhibition than KY 10484 or KY 9159. In KY 10251, which produces both L-threonine and L-lysine, the simultaneous addition of L-threonine and L-lysine hardly inhibited the activity of aspartokinase.
The difference in L-lysine production between strains KY 10440 and KY 10251 could be mainly due to the difference in the susceptibility of the aspartokinase to concerted feedback inhibition by L-lysine and L-threonine. Furthermore, higher levels of L- threonine production in AHV- and AEC-resistant mutants, as compared with the parent AHV-resistant mutants, could be brought about by the genetically determined desensitization of aspartokinase to end product inhibition.
L-Homoserine:
Nara (1972) has reviewed homoserine production. A threonine auxotroph of C. glutamicum produced 13-15 g of L-homoserine per liter and 9 g of L-lysine per liter in a chemically defined medium consisting of 10% glucose, 2% (NH4)2SO4, 400 mg L-threonine per liter, 30 μg biotin per liter, 0.1% K2HPO4, 0.03% MgSO4.7H2O, and 2% CaCO3. In the presence of excess methionine, the yield of homoserine was lowered and that of lysine increased without any change in the total amount produced of both amino acid.
The effect of methionine explained the low yield in natural media. Excess threonine lowered the yield of both amino acids. Homoserine production by threonine auxotrophs has been reported also for E. coli and B flavum. Homoserine production from n-paraffins has also been reported in a threonine auxotroph of Corynebacterium sp. KY 4403.
L-Serine:
Corynebacterium glycinophilum ATCC 21341, produced 10 g of L-serine per liter with a medium containing 2% glycine under favorable conditions. This strain was isolated from a putrefied banana and resistant to a high concentration of glycine. A leucine-methionine double auxotroph, AJ 3414, derived from C. glycinophilum ATCC 21341 produced 14 g of L-serine per liter in a medium containing 3% glycine.
The serine dehydratase activity of these mutants was reduced to 31 and 1.3%, respectively, of the parent strain. The serine hydroxymethyl transferase is induced by glycine, and serine dehydratase is induced by serine formed during fermentation. Chloramphenicol (10 μg per liter) addition at 16 and 48 hr in the culture of the parent strain increased serine production to 11 g per liter compared with 4.6 g per liter in the control culture.
Pseudomonas 3 ab, a facultative methylotropic organism, was incubated for 1 day in a medium containing 1% methanol. After supplementation of this medium with methanol (8 g per liter) and glycine (20 g per liter) and incubation at pH 8.5 for 3 days, 4.7 g of serine per liter were produced. At pH 8.5-9.0, there was only a small degradation of L-serine and glycine.
An attempt to obtain a mutant which produced L-serine directly from cheap carbon sources was not successful although some mutants produced a small amount of L-serine. Formation of L-serine as a by-product by threonine producers was noted. Threonine, homoserine, and glycine contributed to L-serine production by some bacteria. L-Serine production by some bacteria with a medium containing DL-glycerate has been patented.
L-Isoleucine, L-Leucine, and L-Valine:
L-Isoleucine:
It consists of- (1) feedback inhibition of L-threonine dehydratase by isoleucine, (2) inhibition of α-acetolactate (ALA) synthetase by valine, (3) multivalent repression of isoleucine-valine biosynthetic enzymes by all 3 end products of this sequence, i.e., valine, leucine, and isoleucine, (4) feedback inhibition of α-isopropylmalate (IMP) synthetase by leucine, and (5) regulation of intracellular levels of threonine, a precursor of isoleucine.
Thus wild type strains of bacteria do not excrete appreciable amounts of isoleucine in a salts-sugar medium. Microbial production of isoleucine was, therefore, first established by addition of precursors such as α-aminobutyrate (α-AB), D-threonine, and α-hydroxybutyric acid which all escape the regulation.
Fermentation methods using these precursors have been established using various bacteria by several groups and have been utilized industrially. These processes have been reviewed in detail. DL-α- Bromobutyric acid could also be utilized as a precursor for isoleucine production.
In addition to its role as a precursor by deamination to α-ketobutyrate, α-AB stimulates isoleucine production by releasing the feedback inhibition of threonine of threonine dehydratase by isoleucine, and acts as a regulatory factor on acetohydroxyacid (AHA) synthetase, stimulating synthesis of acetohydroxybutyrate (isoleucine precursor) and inhibiting that of acetolactate (valine precursor) in Bacillus subtilis.
D-Threonine induces synthesis of d-threonine dehydratase which is resistant to end-product inhibition by L-threonine, in contrast to L-threonine dehydratase in the species Serratia and Pseudomonas. Hence, D- threonine supplies α-ketobutyrate continuously for isoleucine biosynthesis, avoiding feedback control by isoleucine.
In addition, α-AB formed from excess α-ketobutyrate exerts a stimulating effect on the enzyme systems in these bacteria in favor of isoleucine biosynthesis. In Serratia marcescens No. 1, D-threonine derepresses synthesis of d-threonine dehydratase and AHA synthetase. In an α-AB-resistant mutant derived from S. marcescens No.1, D-threonine dehydratase and AHA synthetase are genetically derepressed. This strain produced 15-16 g of L-isoleucine per liter in the presence of D-threonine.
Recently, processes for L-isoleucine production directly from carbohydrates have been established. Isoleucine hydroxamate (IH) shows false feedback inhibition of L-threonine dehydratase activity. A mutant of Serratia marcescens which is resistant to IH and has desensitized L-threonine dehydratases, and α-AB-resistant mutants which are genetically derepressed for the isoleucine-valine biosynthetic enzyme, produce no isoleucine in a salts-sugar medium in the absence of isoleucine precursors.
A double mutant (IHAV 818) resistant to both IH and α-Ab, isolated from the IH-resistant strain, produced 6-7 g of L-isoleucine per liter in a medium in the absence of threonine. The double mutant lacked both feedback inhibition and repression of isoleucine biosynthesis. Increasing the α-AB resistance led strain GIHVLAv 2795 to produce 12 g of L-isoleucine per liter in a glucose medium.
In Brevibacterium flauum, growth inhibition by α-amino-β-hydroxyvaleric acid (AHV), an analog of threonine, was reversed not only by L- threonine but also partially by L-isoleucine. Threonine-producing mutants, which were isolated as analog-resistant mutants, produced a small amount of L-isoleucine. Strain ARI-129, which is resistant to AHV and which was isolated on a medium supplemented with 2 mg of AHV per ml, produced 11 g of L-isoleucine per liter.
Homoserine dehydrogenase from strain ARI-129 was insensitive to feedback inhibition by L-isoleucine and threonine, while no difference was observed in the activity of threonine dehydratase between strains ARI-129 and 2247A, a wild type parent strain. Another AHV-resistant mutant (ARI-199), which was selected on a plate containing 3 g per liter of AHV, produced 15.1 g of L-isoleucine per liter.
The medium had the following composition – 10% glucose, 5% (NH4)2SO4, 0.15% KH2PO4, 0.05% MgSO4.7H2O,2 ppm Fe2+, 2 ppm Mn2+, 200 μg biotin per liter, 400 μg thiamine HCl per liter, 4 mg Mieki (protein hydrolysate) per liter, and 5% CaCO3. Maximum production of L-isoleucine was obtained after 66 hr. Strain ARI-129 grew slightly better than ARI-199, and the maximum production of L- isoleucine was obtained after 44 hr.
The productivity of strain ARI-129 was more stable than that of ARI-199. The difference between the isoleucine and L-threonine producing mutants was attributed to differences in permeability to L-threonine. In isoleucine-producing mutants, intracellular accumulation of threonine overcomes feedback inhibition of threonine dehydratase by isoleucine because inhibition of threonine dehydratase by isoleucine is competitive with respect to L-threonine.
An O-methyl-threonine-resistant mutant (AORI-126), which was derived from ARI-129, produced 14.5 g of L-isoleucine per liter. The specific activity of threonine dehydratase from strain AORI-126 increased about 2-fold over that of strains No. 2247A and ARI-129, whereas the degree of inhibition of the enzyme by L-isoleucine was the same.
An ethionine (20 mg per ml)-resistant mutant (No. 168) of B. flavum was isolated after mutagenic treatment of strain BB-69, an AHV-resistant mutant which produces L-threonine from glucose in a yield of 13%. Mutant strain No. 168 produced L-isoleucine in a yield of 10%.
Another ethionine (15 mg per ml)-resistant mutant (No. 14083) was derived from B. flavum FAB-3-1, which was resistant to both thialysine (AEC), a lysine analog, and AHV, and produced L-threonine in 11% yield. The mutant (No. 14083) produced L-isoleucine in 12% yield. Production of L- isoleucine by this process using acetic acid as the carbon source reached 33.5 g per liter and a 10% yield.
An L-isoleucine producing mutant (ASAT 372) of Corynebacterium glutamicum was isolated as a thiaisoleucine-resistant mutant from a threo-nine-producing strain having 3 markers- methionine-less, AHV resistance, and thialysine resistance by mutation. ASAT 372 produced 5 g of isoleucine and 5 g of threonine per liter.
This strain was further improved by adding as markers- ethionine resistance, 4-azaleucine resistance, and α-AB resistance. The strain thus obtained, RAM-55, produced 10.6 g of L-isoleucine.
L-Leucine:
A leucine-producing mutant was obtained as a leaky-type isoleucine auxotroph from an α-AB-resistant mutant of Serratia marcescens. The isoleucine auxotroph (No. 149) produced L-leucine by long incubation in a medium lacking isoleucine. S-13 and S-11, partial revertants of strain No. 149, produced 13 g per liter of L-leucine in 48 hr in a medium lacking isoleucine.
The medium had the following composition – 2% glucose, 10% dextrin, 1% urea, 0.1% K2HPO4, 0.05% MgSO4.7H2O, 2% CaCO3. The original α-AB-resistant mutant (Ar 130-1) was derepressed for isoleucine-valine biosynthetic enzymes. Acetohydroxy acid synthetase and transaminases of strains S-3 and S-11 were found to be derepressed 10-20- fold compared with the parent strain No. 149.
Furthermore, α-isopropyl- malate (α-IPM) synthetase, the first enzyme on the leucine biosynthetic pathway of the α-AB-resistant mutant, was also found to be constitutive. Reversion of the isoleucine auxotrophy was accompanied by desensitization of the leucine biosynthetic enzyme. On the other hand, α-IPM synthetase in the wild type strain is repressed by leucine.
L-Threonine dehydratase activity was not detected in leucine-producing revertants by the assay method used. These results indicate that leucine production was due to a lack of both feedback inhibition and repression. In the L-leucine-producing revertant, leucine enzymes catalyzed α-ketobutyrate formation from pyruvate via citramalate, citraconate and β-methylmalate.
Tsuchida et al. (1974B) found an L-leucine producer in the 2-thiazole-alanine-resistant mutants derived from an isoleucine-methionine double auxotroph of Brevibacterium lactofermentum 2256. One of the mutants, No. 218, produced 19 g of L-leucine per liter in a medium having following composition- 8% glucose, 4% (NH4)2SO4, 0.1% KH2PO4 0.04% MgSO4.7H2O, 50 μg biotin per liter, 300 μg thiamine HCl per liter, 2 ppm Fe2+, 2 ppm Mn2+, 40 mg DL-methionine per liter, 20 mg L-isoleucine per liter, and 5% CaCO3. The time course of the reaction is shown in Fig. 17.10.
Strain No. 218 produced 28 g of L-leucine per liter after 72 hr cultivation when 13% glucose was supplied as a carbon source. Maximum production of L-leucine was obtained with a certain degree of oxygen deficiency. When oxygen deficiency degree is defined as rab/KrM, where rab is respiration rate (moles of O2/ml min) of microorganism and KrM (moles of O2/ml min) is maximum oxygen demand rate of microorganism, maximum leucine production by Brevibacterium lactofermentum was attained at an oxygen deficiency degree between 0.8 and 0.9 (Fig. 17.11).
This oxygen deficiency was attained at the redox potential of the medium (Eh, mv) between -200 mv and -220 mv. In a wild type strain of Brevibacterium flavum, α-IPM synthetase is sensitive to inhibition and repression by L-leucine, and AHA synthetase is weakly sensitive to inhibition by valine, leucine, or isoleucine and is strongly repressible by a combination of the 3 amino acids.
On the other hand, in the leucine producer No. 218, the regulatory property of AHA synthetase was almost the same as that of a wild type strain, while α-IPM synthetase was found to be derepressed 3-fold and resistant to feedback inhibition. From these results, both genetic release of feedback inhibition by the mutation causing the 2-thiazolealanine resistance and derepression of AHA synthetase brought about by the mutation leading the isoleucine auxotrophy seem to be the cause of over production of leucine.
An L-leucine production process using an auxotrophic mutant of Corynebacterium glutamicum has also been developed. Mutant No. 190 was found to produce a large amount of L-leucine in the culture medium. The nutritional requirements of the mutant are rather complex but its growth was remarkably stimulated by L-phenylalanine.
Acetate (1.5-3.0%) or pyruvate (3%) stimulated L-leucine production. A histidine auxotrophic derivative, Pα-129, produced twice as much L-leucine as the parent strain, i.e., L-leucine production by the strain reached 16 g per liter in a medium containing 12% glucose, 2.5% CH3COONH4, and other ingredients.
L-Valine:
Uemura et al. (1972) reviewed L-valine production by a fermentation process. Valine-producing bacteria are often found in nature, but processes using auxotrophic or regulatory mutants have also been developed. The addition of certain drugs to the culture medium will induce valine production.
Valine Production by Wild Type Bacteria:
Most of the wild type organisms capable of copious valine production belong to the family Entero- bacteriaceae, especially to the genera Aerobacter and Escherichia.
Optimal carbon:nitrogen ratios for valine production seem to be approximately 100:7 to 100:4. It is noteworthy that heavy metal ions profoundly influence valine production. Ferrous ions seem to be essential for valine production largely because α-acetolactate synthetase (ALSase) and β- hydroxyacid dehydratase are Fe2+-requiring enzymes.
Control of the oxygen supply to the culture medium is another important factor for valine production. Using a selected wild type culture of a strain of Enterobacteriaceae, 12-13 g of L-valine per liter, equivalent to 20% conversion of consumed glucose, could be produced.
Mechanism of Valine Production in Wild Type Bacteria:
Most prototrophic valine-producing bacteria have been shown to belong to the group of bacteria which have 2 α-acetolactate synthetases (ALSases), namely, pH 8 ALSase and pH 6 ALSase. The former enzyme has optimum activity at pH 8 and functions as the initial enzyme in the biosynthesis of L-valine. The second enzyme has optimum activity at pH 6 and performs a biodegrading function leading to acetoin formation, although it can function as a biosynthetic enzyme at lower pH values.
In the bacteria described above, pH 6 ALSase, which is valine-insensitive, is induced by growth in the fermentation medium and functions in the overproduction of valine. This was clearly demonstrated in Paracolobactrum coliforme. Production of valine seemed to be further enhanced due to a lack of ALA decarboxylase in this bacterium.
In Aerobacter aerogenes No. 19-35, studied by Uemura et al. (1972), the pH 6 enzyme was formed inductively in the presence of an optimal concentration of inorganic phosphate or 6-thioI- purines. In addition to P. coliforme and A. aerogenes, Aerobacter cloacae and some E. coli strains belong to the group of bacteria.
Another group of valine-producing bacteria has only pH 8 ALSase. Escherichia freundii, Serratia marcescens, some E. coli strains, Bacillus subtilis, and Brevibacterium ammoniagenes belong to this group. In these bacteria, the pH 8 ALSase was insensitive or less sensitive to feedback inhibition by valine.
When altered regulatory mechanisms are participating, valine overproduction may be understood as a consequence of the extent to which pyruvate accumulates as a catabolic intermediate, the extent to which ALA is efficiently synthesized from pyruvate as a first precursor leading to valine, and to which an amino-donor in the form of glutamic acid is available for a transamination reaction.
Each of these processes is influenced by several external factors, e.g., the presence of amino acids, drugs, and heavy metal ions, partial pressure of oxygen, pH value, and quality and quantity of carbon and nitrogen sources. Hence valine production is dependent on environmental changes, often leading to another mode of fermentation.
Acetohydroxy acid (AHA) synthetase is sensitive to catabolite repression in wild type E. coli B. The synthetase in a streptomycin-dependent mutant of E. coli B which excretes L-valine was relatively resistant to catabolite repression.
Valine Production by Auxotrophic and Regulatory Mutants:
Isoleucine and leucine auxotrophic mutants of C. glutamicum produce a large amount of L-valine in culture broths. Some other auxotrophs produce L-valine in smaller amounts, L-Valine production by an isoleucine auxotroph reached 11 g per liter in a medium containing 7.5% glucose. Addition of leucine and valine markedly increased valine production in a synthetic medium.
Antagonisms among L-isoleucine, L-leucine, and L-valine were observed in growth and valine production. It was speculated that L-leucine and L-valine compete with isoleucine, reversing the inhibition of isoleucine on valine production although permitting protein formation to a certain extent to enable overproduction of valine. Permeation into the cell was considered as a probable step for antagonism by the 3 amino acids.
A mutant of Serratia marcescens resistant to α-AB produced a remarkable amount of L-valine. This overproduction of valine by the mutant was correlated with genetic release from multivalent repression of the formation of isoleucine-valine enzymes.
This was particularly true for the pH 8 ALSase which was sensitive to feedback inhibition by valine to the same extent as the enzyme in the parent strain. In a number of analog-resistant mutants, the best valine producer, strain No. 9, showed high activity of pH 8 ALSase, being also less sensitive to valine inhibition.
A thiazolealanine-resistant mutant of Brevibacterium lactofermentum produced 23.1 g of L-valine per liter in a medium containing 8% glucose. Maximum valine production was attained at an oxygen deficiency degree between 0.5 and 0.7 (Fig. 17.11). This oxygen deficiency degree was attained at the redox potential of the medium (Eh,mv) between -210 mv and -260 mv.
Other Amino Acids:
Alanine:
Direct Fermentation:
Many bacteria, fungi, yeasts, and actinomycetes isolated from natural sources produce alanine in culture media. Prominent producers are Corynebacterium gelatinosum, Brevibac- terium monoflagellum, B. alanicum, B. amylolyticum, B. pentoso-amino-acidicum, Bacillus coagulans, and a mutant strain (13W) of Brevibacterium 22.
In contrast to other amino acids, the alanine produced in these cultures is usually the racemic form. Pseudomonas sp. No. 483 and Micrococcus sodonensis are rare examples of L-alanine producers; D-Alanine is produced by Corynebacterium fascians under certain conditions. An arginine hydroxamate-resistant mutant of Microbacterium ammonia-philum, a glutamic acid producer, also produced a large amount of alanine in a cane molasses medium.
The most common substrate for alanine production is D-glucose, although other carbohydrates are also used. Some alanine producers which utilize n-paraffins as substrate have been reported, but the amounts of alanine produced are small. The concentration of the nitrogen source and the degree of aeration are important factors in producing alanine.
Manganese ions (Brevibacterium pentoso-alanicum) and Zn2+ (Fusarium moniliforme) stimulate L-alanine production. Pyruvate and ammonium lactate increase alanine production with Pseudomonas sp. No. 483. Yields of 40% by a C. gelatinosum strain have been reported.
In Pseudomonas sp. No. 483, alanine is formed from pyruvate by the action of alanine dehydrogenase. Alanine biosynthesis by Arthrobacter sp. 19d in a molasses medium also involves reductive amination. On the other hand, in C. gelatinosum, transamination from glutamate to pyruvate was suggested as the mechanism of alanine formation. Alanine racemase catalyzes conversion of L-alanine to d-alanine yielding an equilibrium racemate mixture.
Enzymic Conversion of L-Aspartic Acid to L-Alanine:
The difficulty in obtaining optically active alanine led to the development of an enzymic process for producing L-alanine from L-aspartic acid. Pseudomonas dacunhae and Xanthomonas oryzae have been selected for the purpose. With P. dacunhae, high L-aspartic-β-decarboxylase activity was obtained by shaking a culture at 30°C in a medium containing ammonium fumarate, sodium fumarate, corn-steep liquor, peptone, and inorganic salts.
For the enzymic conversion of L-aspartic acid to L-alanine, the whole culture broth was employed as an enzyme source. Large amounts of L-aspartic acid (as much as 40% of the broth) were converted stoichiometrically to L-alanine in 72 hr at 37°C. Control of the pH value at around 5.0 was essential for achieving a yield of more than 90%, since otherwise decomposition and racemization of the accumulated L-alanine occurred.
L-Aspartic Acid:
Aspartase activity was exploited for the production of L-aspartic acid from ammonium fumarate. Escherichia coli, E. freundii, and Pseudomonas fluorescens are good sources of aspartase. Escherichia coli are a facultative aerobe, so that cell yield is strongly affected by culture conditions such as the degree of aeration. To avoid glucose repression of aspartase formation, the glucose concentration in the medium should be low, though a small amount of glucose sometimes gives good results causing improved growth of the bacterium. Kitahara’s procedure is as follows-
A large concentration of ammonium fumarate, corresponding to 50 g fumaric acid, was suspended in 100 ml water and subjected to the action of aspartase. Crystals of ammonium fumarate gradually disappeared and were converted to a solution of acid ammonium aspartate. Hydrochloric acid was added to bring the pH value of the solution to 2.8, the isoelectric point of aspartic acid. The solubility of aspartic acid being only 0.6% at this point, the greater part immediately crystallizes out.
ADVERTISEMENTS:
Later Alcaligenes and Pseudomonas ovalis were selected as bacteria which produce L-aspartate from ammonium maleate. The activity of A. faecalis was high when it was grown in an acidic medium due to the permeation of maleate, an inducer of maleate cis-trans-isomerase. Malonic acid was found to be a gratuitous inducer.
Continuous production of L-aspartic acid from ammonium fumarate was attained by employing an enzyme column packed with the immobilized aspartase, a preparation of partially purified aspartase from E. coli entrapped in a polyacrylamide gel lattice. Furthermore, E. coli was used in place of the enzyme.
When a solution of 1 M ammonium fumarate (pH 8.5) containing 1 mM Mg2+ was passed through the immobilized cell column at a flow rate of space velocity 0.8 at 37°C, the highest rate of reaction was attained. L-Aspartic acid was obtained in good yield from the column effluent. The half-life of the immobilized column was 120 days.
L-Proline:
L-Proline, which has a characteristically sweet taste, is not an essential amino acid, but recently medical and food industries have begun to make use of it.
Production of L-proline by fermentation was reviewed by Okumura (1972). L-Proline productivity of an isoleucme auxotroph and a histidine auxotroph of Brevibacterium flavum increased significantly by making them resistant to sulfaguanidine by mutation.
Some auxotrophic mutants of coryneform glutamic acid-producing bacteria, represented by C. glutamicum, produced large amounts of L-proline. These include isoleucine, histidine, and ornithine auxotrophs. According to Araki et al. (1975), a certain base auxotroph of C. glutamicum produced 31 g of L-proline per liter in a medium containing 15% reducing sugars as invert (as cane molasses).
A tyrosine-phenylalanine double auxotroph of Corynebacterium melassecola also produced a fairly large amount of L-proline. Some wild type strains of C. glutamicum also produce significant amounts of L-proline under certain conditions. Production of L-proline by Kurthia catenaforma increased in a serine auxotrophic mutant.
One of the characteristic conditions for L-proline production is a high concentration of ammonium ions. An excessive supply of biotin and unusually high concentrations of magnesium ions were also required for proline production by auxotrophic mutants of coryneform glutamic acid-producing bacteria.
In the case of K. catenafofma, the presence of L-aspartic acid and a high concentration of potassium ions in the medium markedly stimulated L-proline production. L-Glutamic acid was also effective when some surfactants were added to the medium to increase its transport. The nutrients required for auxotrophic mutants should also be limited to an appropriate concentration which is suboptimal for their growth.
Addition of high concentrations of both ammonium and chloride ions to the medium was effective for L-proline production by wild type strains of C. glutamicum. Highly aerobic conditions, a temperature near 30°C, and an initial pH value of 7.0-8.0 were also required for good production of L-proline by C. glutamicum. Adding alcohols increased L-proline production by C. glutamicum KY 9003.
L-Proline production by B. flavum No. 14-5, an isoleucine auxotroph, can be explained on the following basis – An intracellular accumulation of threonine resulting from a blockage of threonine dehydratase activity; availability of a high level of ATP resulting from inhibition of aspartate kinase and of homoserine kinase by the threonine; promotion of glutamate kinase activity by the ATP; insensitivity of one of the two isozymic kinases to proline; and the availability of intracellular glutamate under biotin-rich conditions. However, this explanation may be questioned because many isoleucine auxotrophs deficient in threonine dehydratase are unable to produce a large amount of proline. The mechanism of L-proline production is still waiting to be clarified.
L-Cysteine:
Very recently, two new processes for cysteine production have been reported. High activity of L-cysteine desulfhydrase was found in the bacteria of Enterobacteriaceae. The bacterial cells produced 25 g of L-cysteine per liter from β-chloroalanine and sodium sulfide. The yield increased to 48.5 g per liter (molar conversion from β-chloroalanine is 80.2%) by adding up to 8% acetone to the reaction mixture.
L-Cysteine could be easily recovered as precipitate of cystine by aeration of the mixture at pH 5 after removing hydrogen sulfide in the mixture. Pseudomonas thiazolinophilum AJ 3854 quantitatively converted DL-2-aminothiazoline-4-carboxylate (DL-ATC), an intermediate in the chemical synthesis of cysteine, into L-cysteine.
A part of the L-cysteine produced was spontaneously oxidized to L-cystine by air. In a mutant which lost cysteine desulfhydrase, 95% of DL-ATC was converted into L-cysteine and the concentration of L-cysteine in the reaction mixture reached 31.4 g per liter. ATC-racemase, ATC-hydrolase, and S-carbamylcysteine hydrolase are involved in the conversion.
L-Methionine:
L-Methionine production of Corynebacterium glutamicum KY 9276 (Thr–) was improved by sequential addition of resistance to 5 methionine-analogs. The finally selected strain produced 2 g of L-methionine per liter in a medium containing 10% glucose. Increase of L-methionine production was accompanied by increased levels and reduced repressibility of methionine-forming enzymes.