ADVERTISEMENTS:
The following points highlight the five main components of human immunity system. The components are: 1. Origin and Differentiation of Lymphocytes 2. The Major Histocompatibility Complex 3. Cytokines 4. Antigen 5. Antibodies.
1. Origin and Differentiation of Lymphocytes:
In adults, all kinds of blood cells originate from bone marrow stem cells. These are distinguished into two major types — the myeloid cells and lymphopoietic cells. Precursors of both these types are called haemopoietic stem cells (HSC). The lymphopoietic cells give rise to the lymphocytes. The precursors of T-lymphocytes migrate to the thymus where they multiply and mature in about 72 hours.
The T-cells in thymus are sometimes called thymocytes. Most of the thymocytes (about 95%) produced each day die by a programmed cell death called apoptosis. This elimination of majority of thymocytes is an essential step, because, through this process, those cells which bind to self-antigens are removed. As a result, the surviving T-cells are those that can interact with non-self antigens only.
ADVERTISEMENTS:
The self- antigens are also called the human leucocyte antigens (HLA) which are coded by the genes of the major histocompatibility complex (MHC). The T-cells which bind strongly to the self-antigen molecules are sacrificed and the surviving T-cells from the T-lymphocyte population circulating in the body.
The maturation of T-lymphocytes into immuno-competent cells involves insertion of receptor proteins on their membrane. This receptor is the T-cell receptor (TCR). Each T-cell coming out from the thymus is provided with a unique receptor that can interact with only one specific antigen (more precisely one antigenic determinant). Thus, the T-cell population, taken as a whole, has at least a small number of cells for each of the thousands of different antigens present in the environment. This diversification of T-cells occurs before the T-cells come in contact with the external antigens. The basis of such diversity of T-cells (and also of B-cells).
There are two main types of T-cells — the helper T-cells (TH) and cytotoxic T-cells. These two types have their characteristic surface proteins, CD4 on TH-cells and CD8 on CTL. These surface molecules are also acquired by these cells during differentiation in thymus (Fig. 10.13).
B-lymphocytes are produced and matured in the bone marrow of adults. In birds, B-cells mature in an intestinal gland, called bursa of fabricius, from where these lymphocytes derived their name, B-cell. But the prefix B now refers to the bone-marrow which is believed to be the site of maturation in human.
ADVERTISEMENTS:
During maturation in the bone-marrow, B-cells undergo similar elimination through apoptosis, as the T-cells in thymus. The surviving B-cells are equipped with specific receptors, each having the capacity to bind to a specific antigenic determinant. The B-cell receptors are antibody molecules which are expressed on the surface.
At first, the receptor is an IgM molecule and later an IgD molecule is also added, both of these Ig molecules have the same antigenic specificity. The mature B-cells with the receptors then enter into the blood stream and are transported to the secondary lymphoid organs where they meet the antigen. The diversity of B-cells, like that of T-cells, has a genetic basis. Major function of B-cells is to produce antibodies.
The origin and maturation of B and T-lymphocytes are diagrammatically shown in Fig. 10.14:
2. The Major Histocompatibility Complex (MHC):
The characteristics inherited by an individual include not only the phenotypic traits, but also of different molecules. A group of genes located on human chromosome 6 code for a group of glycoproteins which were initially discovered on leucocytes. As these proteins show antigenic activity in other individuals, they came to be known as human leucocyte antigens (HLA).
The group of genes encoding these proteins forms the major histocompatibility complex, because they were found to be associated with acceptance or rejection of grafted tissues and organs (compatibility/incompatibility of grafts).
The proteins coded by the MHC are now generally known as MHC proteins (in place of HLA). In addition to the determination of histocompatibility in grafts, MHC proteins also perform important functions in immune interactions.
ADVERTISEMENTS:
These latter functions are briefly described below:
The MHC proteins are divided into two classes — MHC Class I and MHC Class II. The genes coding for Class I proteins are located in three clusters called A, B and C and accordingly their products are also known as A, B and C. The Class I proteins have two polypeptide chains, but only one of the two (the larger one) is coded by the MHC Class I genes.
The Class II proteins are coded by three sub-clusters, called DP, DQ and DR. The Class II proteins have also two polypeptide chains and both are coded by the MHC genes. Each of these genes of both classes have many allelic forms.
The combinations of these alleles which are again distributed in six loci produce such great diversity that the chance of having identical sets of MHC genes in two individuals is extremely rare (perhaps J in 10 million) unless the two individuals are identical twins.
ADVERTISEMENTS:
Thus MHC proteins are expressed on the surface of different types of body’s cells. This, MHC Class I proteins are present on all nucleated cells, including, among others, B-cells, T-cells, macrophages, neutrophils, dendritic cells etc.
On the other hand, MHC Class II proteins are present on B cells, macrophages and dendritic cells, but not on other blood cells or body cells. The gross structures of MHC Class I and Class II proteins expressed on cell surface are shown in Fig. 10.15.
It may be noted that the α-chain of MHC Class I is only involved in binding a peptide of a viral or microbial origin. The β-chain is not involved in such binding. In case of Class II MHC proteins, the binding site is generated by both α and β-chains.
The pattern of MHC genes inherited by a person determines the individuality or selfness and a cell or tissue containing a different pattern of the gene products (MHC proteins) is recognized as non-self. The significance of MHC proteins in immunity lies in the fact that antigens must be presented in association with these self proteins to be recognized by the immune cells, like T-cells, because these cells are unable to recognize free antigens. Thus, when a virus infects a body cell, the virus is degraded and its peptides which act as antigenic determinants are associated with MHC Class I proteins of the infected cell and displayed on its surface.
ADVERTISEMENTS:
A cytotoxic T-cell with a TCR and CD8 protein on its surface then can recognize and bind to the virus-infected body cell and destroys it. This involves a dual recognition, one between the antigenic determinant and TCR and the other between CD8 and MHC proteins of the infected cell. This ensures that the two reacting cells are from the same body.
MHC Class II proteins are involved in antigen presentation by the antigen presenting cells, like macrophages, dendritic cells as well as by B-cells. These cells process the antigens by breaking them to release antigenic determinants (peptides) which are complexed with MHC Class II proteins and presented on their surface.
The MHC Class II proteins complexed with antigenic determinants leads to interaction with generally the T-helper cells. For example, the binding between a B-cell displaying an antigenic determinant on MHC Class II protein and a TH-cell results in activation of the B-cell.
ADVERTISEMENTS:
Antigen presentation and interaction between an antigen presenting cell and a T-cell are diagrammatically represented in Figs. 10.16A and 16B:
3. Cytokines:
Cytokines are a large group of small glycoproteins, secreted by a variety of cells of the body. More than 60 cytokines are now known to function as signal molecules communicating between different types of cells. They have multiple functions including cell proliferation and activation, cell differentiation, chemo-taxis, etc. A particular cytokine-producing cell can produce one or more different types of these chemical mediators. Also, a specific cytokine may have opposing effects, like stimulation and inhibition depending on conditions.
Although such chemical mediators are generally known as cytokines, they have also specific names depending on the types of producing cells. Thus, cytokines produced by leucocytes and used for intercommunication among them are called interleukins. Cytokines produced by monocytes and their derivatives, macrophages, are known as monokines.
Cytokines produced by virus-infected cells are interferon’s. A group of cytokines, called chemokine’s, have been discovered in more recent times. Chemokine’s are produced by many types of cells. They have different functions including chemo- attraction of neutrophils to an infected site and activation of neutrophils.
ADVERTISEMENTS:
Among other cytokines are tumour necrosis factor (TNFP) which is cytotoxic to tumour cells and it activates phagocytic activity. Another cytokine, colony stimulation factor (CSF) induces development and differentiation of erythrocytes and leucocytes from stem cells.
Some representative cytokines and their functions are summarized in Table 10.3:
Abbreviations: CTL: Cytotoxic T-cells; NK: Natural Killer cells; PMN: Polymorphonuclear cells; TH – T-helper cells.
4. Antigen:
An antigen is an agent which is recognized by the body as non-self and can provoke an immune response. It may be a chemical substance, like a protein or a polysaccharide, or it may be a biological entity, like a microbe or virus or a larger parasite. The immune response provoked by an antigen challenges the foreign agent by producing antibodies and specialized T-cells which remove the harmful effects of the antigen.
As any foreign agent can act as an antigen, the list of antigens is endless. Very often an entire organism can act as an antigen. Obviously, an organism contains many different substances, each of which can act as an antigen. Many biological products — milk, egg albumin, bee venom, snake venom, pollen grains etc. — can act as antigens.
ADVERTISEMENTS:
Again, different parts of a single bacterial cell, like flagella, pili, lipopolysaccharides of outer membrane of Gram-negative bacteria, the capsular polysaccharides, the cell membrane, cytoplasmic proteins, exotoxins etc. can each have antigenic activity and they can each provoke a different immune response. Such antigens are given separate names. For example, the flagellar antigens are called H-antigens, capsular antigens are known as K-antigens, outer membrane antigens as O-antigens, etc.
(a) Properties of antigens:
Two basic properties of antigens are their immunogenicity and antigenic specificity. The first one refers to their ability to provoke the immune response. That is why antigens are also called immunogens. The second property refers to the ability of an antigen to combine with only that antibody and T-cells which are produced in the immune system in response to the specific antigen. Although these two properties are interlinked, they are distinct from each other, as it will be presently explained.
The chemical substances which can stimulate immune response include biopolymers, like proteins and polysaccharides. Nucleic acids and lipids in general are non-antigenic; but when they are joined to proteins and polysaccharides, the conjugated molecules become antigenic.
Antigens are always macromolecules having a molecular weight generally above 10,000 Daltons. It is also essential that antigens reach the immune system with their immunogenic property unaltered. For example, proteins and polysaccharides taken as food are not antigenic, because they are digested into their monomers in the GI tract before they are absorbed into tissues.
Not only a biological cell or a virus particle can possess many different antigens, a single protein molecule can provoke the formation of different antibodies and reactive T-cells. Although these antibodies and T-cells arise through antigenic response of a single protein molecule, they cannot recognize the whole antigenic molecule as such, but only specific regions or units of it.
These reactive units of an antigen are called antigenic determinants or epitopes. The total number of epitopes may vary depending on the complexity of the antigenic molecule. Thus, protein molecules are made from 20 different amino acids assorted in innumerable ways.
They have a much greater number of epitopes than a polysaccharide molecule which contains a single or a few different monomers repeated many times. In a protein antigen, a group of 3 to 6 amino acids constitutes an epitope. In a polysaccharide the number is 5 to 6 sugar residues. The characteristic structure of an epitope is produced by folding or conformation of the antigenic molecule.
An important feature of the immune cells that needs to be emphasized is that any one B-cell or one T-cell can recognize only a single epitope of an antigen and can interact with it using specific receptors on their surfaces. The epitopes must be complexed with MHC proteins to be recognized by a T-cell.
It follows, therefore, that an antigen containing a multiple of epitopes provokes formation of many different clones of B-and T-cells, each clone capable of interacting with a separate epitope. Also, such a clone of B-cells is transformed into plasma cells which produce antibodies having the ability to bind only a single epitope. Thus, a single antigen with multiple epitopes elicits formation of a mixture of antibodies (polyclonal).
It should be noted that an epitope itself is not immunogenic, but they are essential for interaction between the immune cells and the antigen which carry the epitopes. Thus, epitopes confer the antigenic specificity, without being immunogenic. This can be best understood from the behaviour of some email molecules which are foreign to the immune system.
A well-known example is dinitrobenzene which is non-immunogenic, but when it is covalently, linked to serum protein the conjugate can elicit an immune response. The antibody formed can combine with the conjugated protein, but not with the carrier protein without dinitrobenzene. Evidently, dinitrobenzene acts like an epitope which determines the antigenic specificity of the conjugate protein. These small molecules are known as haptens. Haptens, like epitopes, determine antigenic specificity, but are not immunogenic. Thus, immunogenicity and antigenic specificity are distinct from each other.
(b) Super antigens:
Normally, the antigens are recognized by immune cells after they are broken down to liberate their epitopes which are complexed with MHC proteins and displayed on the surface of the antigen- presenting cells. In contrast, the super antigens do not need to be processed and they bind directly to the MHC proteins of Class II on the surface of the antigen-presenting cells.
The super antigens evoke a much stronger immune response than normal antigens by stimulating the TH-cells to liberate cytokines. An example of a super antigen is the staphylococcal enterotoxin causing food poisoning.
(c) Alloantigen’s and blood groups:
Alloantigen’s are substances present in some individuals which act as antigens in other individuals of the same species and provoke an immune response. They are also sometimes called iso-antigens. Typical alloantigen’s are the polysaccharide antigens of the erythrocytes which are responsible for the ABO blood groups in humans, as also other blood groups. The MHC proteins, also called-human leucocyte antigens (HLA), are other examples of alloantigen’s.
The ABO system of human blood grouping is based on the alloantigen’s present on the surface of erythrocytes. There are also alloantibodies in the serum which can react with the alloantigen’s. For example, a person with blood group A has alloantigen A on erythrocytes and an antibody against alloantigen B in serum. In the same way, a person with blood group B has alloantigen B on erythrocytes and an antibody against alloantigen A in serum.
In the ABO system there are four groups, A, B, O and AB. In a person with AB group, erythrocytes have both A and B alloantigen’s, but no anti-A or anti-B antibody in serum. On the other hand, a person with O group has neither of the alloantigen’s on erythrocytes, but has both anti-A and anti-B antibodies in serum.
The ABO blood groups are controlled genetically by three alleles (alleles are alternative forms of a gene). These alleles are designated as A, B and O of which A and B are dominant over O. Thus, six possible genotypes are possible : AA, AO, BB, BO, AB and 00 and the corresponding phenotypes (i.e. blood groups) are A, A, B, B, AB and O, respectively.
The relationship of these with alloantigen’s and alloantibodies are shown in Table 10.4:
The importance of blood groups comes in consideration when a person needs transplantation of blood or other tissues. Due to the presence of the alloantigen’s and the alloantibodies, blood transfusion from one person to a recipient is safe only when their blood groups are compatible. Otherwise transfusion may lead to serious reactions caused by clumping and haemolysis of the transferred blood cells in the recipient’s vascular system.
Transfusion reactions occur because the alloantigen’s of the erythrocytes of the donor react with the alloantibodies of the recipient’s serum resulting in clumping and eventual haemolysis. The alloantibodies are also known as iso-haemagglutins, because they cause agglutination of the erythrocytes.
Safe transfusion is possible between persons of the same blood group, as well as if a donor has O group (universal donor). On the other hand, a person with AB group can receive from persons of any other group (universal recipient) because the serum does not contain any alloantibodies.
Another important human antigen used in blood grouping is the Rh-antigen. On the basis of the presence and absence of this antigen, two groups — Rh-positive and Rh-negative — are recognized. Landsteiner and Wiener (1940) discovered that antibodies produced in rabbits by injecting erythrocytes of Rhesus monkey caused agglutination of erythrocytes of about 85% of human population.
The antigens present in such persons was called the rhesus-or Rh-antigen. The erythrocytes of the remaining 15% of the population did not agglutinate and such persons were designated as Rh-negative. When the Rh-positive erythrocytes enter into the blood of an Rh-negative person, the introduced erythrocytes provoke the formation of an Rh-antibody in the recipient. This may lead to complications in fetus from parents where the male partner is Rh-positive and the female partner is Rh-negative.
Besides, ABO and Rh blood groupings based on alloantigen’s, several other systems of grouping, also based on other alloantigen’s in humans, have been discovered later. These include the MNS system, Kell and Duffy system etc.
Another group of alloantigen’s, the human leucocyte antigens (HLA) are present not only on the white blood cells as the name suggests, but also in all nucleated body cells. These antigenic proteins are encoded by six genes of the major histocompatibility complex (MHC), each of these genes having many allelic forms.
Besides their role in antigen presentation, alloantigen’s play very important role in tissue transplantation. Unless the combinations of alloantigen’s of MHC group match, the tissue grafted from a donor is recognized as non-self by the recipient’s immune system and is rejected.
5. Antibodies:
Antibodies are soluble glycoproteins belonging to the class gamma-globulins. They are produced in the body by specialized cells in response to antigens and are important constituents of the immune system. Hence, they are also known as immunoglobulin’s (Ig). At any given time, these proteins may account for about 17% of the total proteins present in blood.
The antibodies present in the body are of many different specificities and they belong to a number of different classes having different functions, but all the functions are directed towards protecting the body from the injurious effects of the antigens that stimulate the formation of the antibodies. The type of protection afforded by antibodies is known humoral immunity.
(a) Structure of antibody molecules:
The basic unit of an antibody molecule consists of four polypeptide chains of two types which are bound to each other by disulfide (-S-S-) bonds. Of the four polypeptide chains, two are longer than the other two. The two longer chains are identical to each other and are known as heavy (H) chains, and the two shorter chains are also identical to each other and are known as light (L) chains.
One L-chain of the pair is bound to one H-chain by a disulfide bond and the other L-chain to one H-chain by a similar bond. The two H-chains are also linked by disulfide bonds. The four chains thus bound, build a Y-shaped structure as shown in a simplified form in Fig. 10.17. This four-chain basic structure represents a monomer of an Ig-molecule. In some antibodies, two such monomeric units may be joined to form a dimeric Ig molecule. In still others, five monomers may be joined to form a pentameric molecule.
There are still other types of structural variations in Ig molecules. The L-chains are of two main types — kappa (k) and lambda (λ). Similarly, the H-chains can be of five different types — alpha (α), delta (δ), gamma (γ), mu (μ) and epsilon (ϵ). These chains differ in the total number of amino acid residues. For example, kappa and lambda light chains have 211 and 217 amino acid residues (M.W. about 23,000 Daltons). The H-chains are about twice as long as the L-chains and have 450-550 amino acid residues in different types.
The Y-shaped Ig molecule is a flexible structure and it can fold on itself along a hinge situated in the heavy chains near the middle (shown in Fig. 10.17). The hinge gives segmental flexibility to the Ig molecule. The amino terminal ends of all the four chains are situated at the ends of the two arms. These ends of the four chains also constitute the site where the antigens bind. Thus, each four-chain molecule has two antigen-binding sites.
The H and L chains form several intrastrand loops stabilized by disulfide bridges. The loops are almost at uniform distances and each of them contains about 60 amino acid rests (Fig. 10.18). Also, the H-chains and L-chains are divided into several segments, commonly called domains. The antigen- binding domains of the H and L chains are designated as VH and VL, respectively, where V stands for variable.
The domains are so named because amino acid sequence of these V-domains is variable for different antibodies having different antigenic specificities. The V-domains of both H-and L-chains fold characteristically, determined by their amino acid sequence to form the binding site of the epitopes of an antigen.
The other domains of H and L polypeptides are the constant domains, CH and Q., respectively. In these constant domains, the amino acid sequences of H and L chains of the same class of Igs are more or less fixed. In the L-chain, there is only one constant domain (CL), but in the H chain, the constant domain is subdivided into several subdomains — CH1, CH2 and CH3 (Fig. 10.18).
When a monomeric Ig-molecule is treated with a proteolytic enzyme, like papain in presence of cysteine, the molecule is cleaved into three fragments. Two of these are identical and they retain their antigen binding sites. The third fragment can be crystallized and it is without antigen-binding capacity, but it can bind complement. The antigen-binding fragments are called Fab and the crystallizable fragment is called Fc.
The Fab fragments originate from the two arms of the Ig molecule, while the Fc fragment from the stem portion. Thus, the papain treatment cleaves an Ig molecule in the middle near the hinge portion. The Fab fragments consist of the entire L-chains and about half of the N-terminal portions of the H-chains. The Fc-fragment consists of the rest of the H-chains only (Fig. 10.19).
The function of the Fab fragments is to bind to specific antigenic determinants (epitopes) present on the antigen. The amino acid sequences of the variable domains of H and L chains determine the specificity i.e. to which epitope it binds. The variable domains of both chains (H and L) constitute the binding site.
As each Fab fragment can bind to a single epitope, the intact Ig molecule is capable of binding two epitopes of the same specificity. The antigen-binding capacity of an Ig-molecule is known as its valence. So, a four-chain monomeric Ig molecule is bivalent.
The Fc-fragment contains oligosaccharide side chains linked to the constant domain of the H-chains. An important function of the Fc fraction is to bind complement when the Ig molecule has formed a complex with an antigen by binding with its antigen-binding sites. The binding of complement to the Fc region leads to activation of the complement system resulting in destruction of a microbial antigen. The Fc region can also bind to certain types of cells, like macrophages.
(b) Classes of Ig molecules:
Ig molecules are made of two types of L-chains, k and λ, and five types of H-chains, α, δ, y, μ and ϵ. Ig molecules are classified into five major classes depending on which type of H-chain is present. These five major classes of Igs are IgG, IgA, IgM, IgD and IgE which have H-chains of y, α, μ, δ and ϵ types respectively.
So far as the L-chain is concerned, all the five classes may have an L-chain of either k or X type. So, the nature of the L-chain does not count in the classification of Ig molecules. About 60% of the human Ig molecules have a k type L-chain.
Of the five classes of Ig molecules, IgG, IgD and IgE consist of four-chain monomeric units with two antigen-binding sites (bivalent). IgA is a dimeric molecule with two monomers joined to each other by an extra polypeptide chain, called a J-chain.
There is also another protein associated with IgA which enables it to be secreted into body fluids. This component of IgA is called the secretory component. IgA is tetravalent. IgM is pentameric consisting of five monomers joined to each other by a J-chain. It is decavalent having 10 antigen binding sites.
The structures of the different classes of Ig molecules are shown in Fig. 10.20:
(c) Properties of different classes of Ig-molecules:
Different classes of Ig molecules are characterised by distinctive properties as well as functions. Also, these classes differ considerably in their affinity towards antigens. Affinity refers to the firmness of binding between an antigen-binding site of an Ig-molecule and an epitope of an antigen.
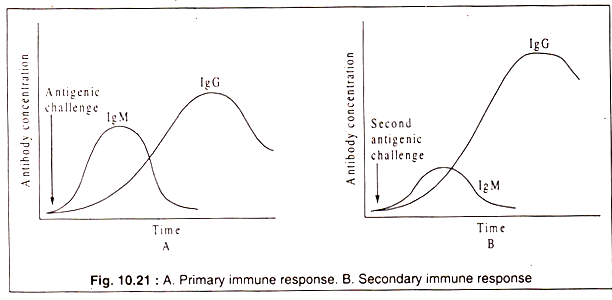
Generally, antibodies produced initially in an immune response bind rather loosely to an antigen than those produced later. The first antibody that appears in the primary immune response belongs to the IgM class. It is soon outnumbered by antibodies of the IgG class.
Probably, the different classes of Ig molecules evolved to meet the challenge of pathogenic agents which enter the body through different routes.
Some important characteristics of the five classes of Ig molecules are summarized in Table 10.5:
The characteristics of the five major classes of Igs are discussed in more details below:
IgG:
Quantitatively, IgG is the most abundant immunoglobulin present in blood, lymph and in extravascular spaces of the body constituting about 80% of the total immunoglobulin’s. IgG molecules can diffuse through the endothelium of blood vessels into the tissues. It is also the only Ig that can cross the placenta and enter into fetus giving it passive immunity.
It can fix complement to its Fc domain and by activating the complement cascade it can destroy invading microbes. There are four subtypes of IgG (IgG1, IgG2, IgG3 and IgG4) which have minor differences in the amino acid sequences of their H-chains as well as differences in functional activities. IgG level in serum increases after 10 to 14 days from the beginning of the immune response following IgM which is produced initially in response to an antigen.
But a subsequent challenge with the same antigen elicits a rapid and higher increase in IgG level. This is called a secondary response and it is due to the presence of memory cells produced during the primary response. These memory cells circulate in the vascular system and immediately recognize the antigen and stimulate the B-cells to produce antibodies.
The primary and the secondary responses are graphically represented in Fig. 10.21:
IgM:
This Ig is the first to appear in an immune response. It is a pentameric molecule and has a large size. Due to its large size, it cannot move through the wall of blood vessels and it remains restricted to the blood. The five four-chain units are bound by disulfide bridges and also by a J-chain which has a molecular weight of 15,000 Daltons. IgM is also the first antibody on the surface of B-cells where they act as receptors of antigens. On the B-cell surface IgM remains as monomer, while in the blood it is present as a pentamer. For having 10 antigen-binding sites, the pentameric IgM is very effective in removal of microbial antigens.
IgA:
IgA is a secretory antibody. It forms 10 to 15% of the total immunoglobulin’s in the serum, where it occurs as a monomer (M.W. 170,000 Daltons). However, it is the most abundant antibody in the mucosal membranes of the respiratory tract, GI tract and urogenital tract, as well as in the body secretions, like saliva, mucus, tears, breast-milk and colostrum. In these secretions, IgA is present in dimeric form (M.W. 420,000 Daltons).
The higher molecular weight of secreted IgA is due to the presence of an additional J-chain and a secretory component. The secretory component is a polypeptide which is added to the dimer when it passes through the epithelial cells of the mucous membrane. This polypeptide protects IgA from the proteolytic enzymes. IgA is mostly synthesized by plasma cells located in the mammary glands and salivary gland. The major function of IgA is against the microbes which enter through mucous membranes.
IgD:
This antibody forms a minor component of the total immunoglobulin’s in serum. It is mostly restricted to the membranes of B-cells, where they are present along with IgM during the early stages of B-cell differentiation. They mainly function as antigen receptors. When a B-cell contains both IgM and IgD on its surface, it can internalize and process the antigen and the processed antigen is presented to T-helper cell which then helps the B-cell to multiply and to differentiate into an antibody producing plasma cell. However, the exact role played by IgD remains uncertain.
IgE:
IgE is present in very low concentration in serum, but it plays an important role in acute inflammatory response and in causing hypersensitive reactions (allergy). IgE produced by an antigenic stimulation can bind to specific receptors on mast cells and basophils with their Fc domains. When such IgE-bound mast cells and basophils encounter the same antigen subsequently, the antigen binds to the antigen-binding sites of IgE.
This causes the mast cells and basophils to release histamine and other chemical substances from their granules resulting in the allergic reactions, like asthma, hay fever etc. The release of these chemical mediators has also a beneficial role in developing an acute inflammation, because through this process, the body — to removes the harmful effects of the antigen.
(d) Monoclonal antibodies:
Any one B-cell can interact with a single antigenic determinant or epitope. A microbial antigen or even a single molecule of a protein generally have several too many epitopes. When such an antigen stimulates antibody production, the synthesized antibodies necessarily represent a mixture of antibodies. Such a mixture of antibodies is called polyclonal, because-they are produced by different clones of B-cells. A clone is defined as descendents of a single cell.
Evidently, the total B-cell population of the body is composed of an innumerable number of different clones, each clone of B-cells having the ability to interact with a single epitope. The antibody produced by a single clone of B-cells is called monoclonal antibody.
Monoclonal antibodies are, therefore, identical immunoglobulin molecules and completely homogenous with regard to their class, affinity and specificity. They have identical molecular structure of their antigen-binding sites. In Fig. 10.22, it is shown how an antigen with several epitopes gives rise to polyclonal antibodies. It can be seen that polyclonal antibodies are nothing but a mixture of different monoclonal antibodies.
There would be no difficulty in getting monoclonal antibody in vitro, if it is possible to grow a single B-cell artificially in a culture medium. But, unfortunately, B-cells or, for that matter, T-cells cannot be propagated in culture indefinitely. After a few divisions, they stop dividing.
However, like other cells of body, lymphocytes also may become transformed into malignant cells (cancer cells). Such lymphocytes are then called myelomas. A cancerous B-cell, like other malignant cells, can grow indefinitely in culture.
In 1975, Kohler and Milstein developed a technique for production of monoclonal antibody in the laboratory. The conceptual basis of this technique was to isolate a normal antibody-producing B-cell and to confer on it the property of indefinite proliferation in cell culture.
This was achieved by fusing the normal B-cell with a myeloma originating from a B-cell. The fusion product is called a hybridoma (a hybrid of a normal B-cell and a malignant B-cell). A hybridoma can grow indefinitely in culture, by virtue of its myeloma constituent and produces a monoclonal antibody specified by the B-cell constituent.
The technique has proved very successful in production of monoclonal antibodies artificially in adequate quantities for medicinal and research purposes. Kohler and Milstein were awarded (with N. K. Jerne) the Nobel Prize in 1984 for their outstanding contribution.
Because of the classical importance of the discovery, the experimental procedure followed by them is briefly described below:
In developing the cell-line for monoclonal antibody production, Kohler and Milstein mixed spleen cells containing normal B-cells and plasma cells of an immunized mouse with cultured unimmunized mouse myeloma cells in presence of polyethylene glycol (this agent induces cell fusion) to obtain hybridomas.
Naturally, the hybridomas were produced through fusion of different clones of B-cells or plasma cells with the myeloma cells. It was necessary therefore, to isolate a single kind of hybridoma from the mixture which would produce an antibody with a single specificity.
Moreover, it was also necessary to eliminate the unfused myeloma cells. To facilitate elimination of the unfused myeloma cells, the scientists used a mutant cell-line of myeloma which was deficient in the enzyme, hypoxanthine — guanine phosphoribose transferase (HGPRT).
The deficient mutant is killed by a drug, called aminopterine which blocks nucleoside synthesis. While the unfused myeloma cells which are deficient in HGPRT are killed by the drug, the hybridomas survive, because they possess the normal gene coding HGPRT of the B-cell. For elimination of unfused myeloma cells, the mixture of B-cells, hybridomas and unfused myeloma cells was transferred to a selective medium containing among other usual ingredients for supporting growth, also hypoxanthine, thymidine and aminopterine (the inhibitor). This medium, known as HAT-medium, does not allow growth of the mutant myeloma cells.
The unfused B-cells or plasma cells were eliminated by their natural decay. Therefore, what remained now was a mixture of hybridomas which could grow producing different clones of hybridomas. The final step involved isolation of a single clone of hybridomas from a mixture, each of which was capable of producing a different monoclonal antibody. The mixture of hybridomas was separated by growing it in wells of microtitre plates, so that each hybridoma produced progeny cells.
The presence of a desired monoclonal antibody was tested by standard methods, like ELISA, RIA etc. The selected hybridoma line was then propagated in cell culture or in an experimental animal. In a suspension cell culture, a hybridoma can grow to reach a density of 1 x 104 to 1 x 106 per ml and secrete about 10|ig monoclonal antibody per ml. In experimental animal a higher concentration may be obtained.
An outline of the experimental procedure is shown in Fig. 10.23:
Uses of monoclonal antibodies:
The usefulness of monoclonal antibodies depends primarily on their high specificity to react with epitopes present on antigenic molecules or antigenic cells or virus particles. Thus, they can serve as excellent agents for detection of target molecules or biological agents.
They have been used as diagnostic tool for detecting infections caused by Chlamydia which are obligate intracellular parasites and for identification of specific strains, such as those of streptococci. Monoclonal antibody is used in pregnancy test for detecting human chorionic gonadotropic hormone which is excreted only in urine of pregnant women. Monoclonal antibodies also find applications in basic research, such as detection and locating specific proteins in complex structures, like that of ribosomes.
Monoclonal antibodies are used to overcome the problem of rejection of transplanted organs. In such rejection, cytotoxic T-cells play an important role. Monoclonal antibodies which specifically react with T-cells have been found to suppress T-cell activity.
Another important prospective target area of application is in the treatment of cancer. Cancer cells are abnormal body cells and they have specific antigenic molecules on their surface which are absent in body’s normal cells. Monoclonal antibodies raised against the tumour specific antigens (TSA) should bind to them.
If monoclonal antibodies are chemically combined with anti-cancer drugs or radioactive compounds, they would carry the killing agent directly to the target cancer cells and destroy them without affecting normal cells. Such antibody-drug combinations have been designated as immunotoxins.
Even monoclonal antibodies alone (without the toxic drug) have the potentiality to destroy tumour cells by causing lysis through activation of the complement system. Some specific monoclonal antibodies have actually been recommended for human application against certain types of cancers and have yielded encouraging results.
Monoclonal antibodies specific against TSA have been used for removing (purging) cancer cells from bone marrow during autologous bone marrow transplantation. Cancer patients often require prolonged administration of cytotoxic chemotherapeutic drugs and exposure to radiations. These treatments also affect the stem cells of bone marrow from which blood cells are differentiated.
An accepted procedure is autologous bone marrow transplantation. Bone marrow of the patient is taken out and treated with tumour-specific monoclonal antibodies for removing cancer cells, if any present in the bone marrow.
The patient is then treated with anticancer drug and/or irradiation for a sufficiently long period to kill all cancer cells. The cancer cell-free bone marrow is then transplanted to allow stem cells to form new blood cells.
This is schematically shown in Fig. 10.24:
One of the limitations of using commercially available monoclonal antibodies for human application is that these mouse antibodies sometimes produce immune reactions (allergy). Attempts have been made to synthesise chimeric monoclonal antibodies by genetic engineering techniques.
These antibodies contain partly mouse and partly human immunoglobulin’s e.g. the antigen-binding variable domains of H and L chains are coded by mouse genes and the constant domains by human genes. These chimeric molecules are more acceptable to the human immune system.
In more recent times, it has been possible to replace mouse immunoglobin-coding genes by human genes in mouse, so that mouse B-cells produces human immunoglobulin’s. When such B-cells are fused with myeloma cells, the hybridomas produce human immunoglobulin’s as monoclonal antibodies.