ADVERTISEMENTS:
In this article we will discuss about cell-mediated immunity.
While humoral immunity protects the body from extracellular pathogenic agents and soluble antigens like toxins, cell-mediated immunity is directed against intracellular pathogens. The infected body cells are recognized by the immune cells and are destroyed.
Also, abnormal body cells, like cancer cells, are recognized as non-self due to the presence of tumour-specific antigens on their surface and are destroyed by the cell-mediated immune system. Similarly, transplanted cells and tissues become target of attack of this type of immunity.
ADVERTISEMENTS:
Cell-mediated immunity is due to the activity of T-lymphocytes, just as humoral immunity is due to the activity of B-lymphocytes, though other cells of immune system are also involved.
(i) T-Lymphocytes (T-Cells):
T-cells, as also B-cells, originate from lymphopoietic stem cells. The precursors of T-cells proliferate, differentiate and are matured in thymus gland. Within the thymus gland, they are generally called thymocytes. In the thymus gland, the thymocytes proliferate in large numbers and are differentiated, but majority of them are eliminated by apoptosis.
Such selection is based on the ability of T-cells to bind to self-antigens. The surviving T-cells are those which bind weakly to the MHC proteins. These cells then migrate via blood stream to the secondary lymphoid organs, like lymph nodes and spleen. But before they leave the thymus, each T-cell acquires a specific acceptor for an antigenic determinant.
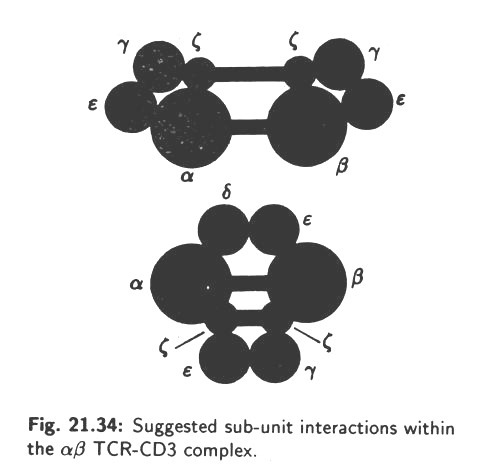
This is called the T-cell acceptor (TCR). Also, during differentiation in the thymus, T-cells acquire the CD4 or CD8 surface proteins which confer their functional behaviour (see Fig. 10.13). The T-cells come across the antigens for the first time in the secondary lymphoid tissues and clonal selection occurs, as it happens also in case of B-cells.
ADVERTISEMENTS:
There are two main functional types of T-cells which are T-helper (Th) cells and cytotoxic T-cells (CTL). These two types are distinguished by possession of different surface proteins, viz. CD4 protein on TH-cells and CD8 protein on CTL. In addition to these two main types, there are two other types. These are delayed hypersensitivity T-cells (TD) which also possess CD8 protein, and suppressor T-cells (Ts) which possess CD4 protein.
The major function of cell-mediated immunity is to kill an infected body cell. This function is carried out by the CTL. On the other hand, the TH-cells help in activation of other cells. The function of Ts and TD cells are less well-known.
The CTLs bind with the help of CD8 protein to the infected body cells which express the antigenic determinants embedded in MHC Class I protein. A CTL recognizes the antigenic determinant with the help of its cognate TCR, while the CD8 binds to the MHC Class I protein. On the other hand, a TH-cell binds with its TCR to an antigen-presenting cell, like a macrophage or a dendritic cell or a B-cell which expresses the antigenic determinant embedded in MHC Class II protein. In this case, the CD4 protein of TH-cell binds to the MHC protein.
In both CTL and, TH-cell, recognition of the specific antigenic determinant is made by their cognate TCRs and the binding is stabilized by CD8 and CD4 proteins, respectively. In addition to CD8 and CD4, other surface proteins are involved in effective binding with their target cells.
(ii) T-Cell Receptor (TCR):
Just as each B-cell possesses a specific antigen-receptor, so also every T-cell — irrespective of its type — has a receptor, called a T-cell receptor (TCR) which can recognize and bind to a specific antigenic determinant. A TCR can recognize an antigenic determinant only when it is complexed with an MHC protein. In contrast; a B-cell, in which the antigen-receptor is an Ig molecule of one class or other, can recognize both cell-bound as well as free antigens.
A TCR is a heterodimer consisting of two polypeptide chains, α and β, having molecular weights of 50 and 39 kilo Daltons, respectively. The two chains are joined to each other by disulfide bonds and both are anchored into the membrane of the T-cell. Each chain has a variable domain and a constant domain. The variable domains of the α and β chain constitute the antigen-binding site TCR. Thus, it is seen that TCRs resemble the Ig molecules in many respects and actually they belong to the immunoglobulin super-family. At the same time, TCRs differ in several respects from Igs.
For example, TCRs are always anchored to T-cell membrane, and they are never found free in the plasma or body fluids as Igs are. Again, TCRs can interact only with antigens complexed with MHC proteins of target cells, whereas Ig molecules can react with both cell-bound or free antigens.
The structure of a TCR is shown in Fig. 10.41:
(iii) T-Cell Diversity:
As one particular T-cell can recognize only one specific antigenic determinant, the total T-cell population necessarily represents an enormously large number of diverse clones of these cells. T-cell diversity is due to the T-cell receptor. The basic mechanism of creating this diversity is similar to that which causes antibody diversity. Just like the H- and L-chains of Ig molecules, the α- and β-chains of TCR contain different domains.
ADVERTISEMENTS:
Each of these domains is coded by multiple DNA segments in the germ-lines. About one million different β-chains and 25 different α-chains are present, yielding about 25 x 106 different TCRs. The α-chains are made of V, J and C domains, and P-chains possess V, D, J and C domains. The genes coding these domains of polypeptides are located in human chromosome 7 (for α-chains) and chromosome 14 (for β-chains).
The gene clusters for the two chains are shown in Fig. 10.42:
As in case of immunoglobulin’s, gene rearrangement by random selection of DNA segments of one V-segment, one J-segment and the C-segment leads to formation of the gene for α-chain. In case of β-chain, there is one extra domain D coded by two DNA segments, D1 and D2. It can be seen that the genes controlling the β-chain are scattered (unlinked).
ADVERTISEMENTS:
Rearrangements of TCR genes starts soon after the precursors (lymphopoietic stem cells) enter into the thymus gland. Diversity arises primarily through random somatic recombination of the V, J, D and C segments of DNA in case of the β-chains, and V, J and C segments in case of α-chains. But further diversity is also added by imprecise recombination (junctional diversity), as it has been explained in case of antibody diversity.
After TCR polypeptides have been synthesized, they are expressed as TCR on the surface of the thymocytes. Those T-cells which bind strongly to the MHC proteins (self-antigens) are eliminated by programmed cell-death (apoptosis). The surviving T-cells (less than 5% of the total thymocytes produced from stem-cells) which bind weakly to self-antigens then come out from thymus as immuno-competent cells.
(iv) T-Cell Activation:
Cell-mediated immunity, also known as cellular immunity, provides protection against intracellular pathogens, mostly viruses, but also some bacteria and fungi which are able to grow within infected body cells. The immuno-potent T-cells destroy such infected cells, thereby making the pathogens open to attack by the antibodies.
ADVERTISEMENTS:
The key-role in killing the infected body cells is played by the cytotoxic T-cells (CTLs) but for doing it, CTLs need to be activated. In this process of activation, TH cells are actively involved. On the other hand, TH-cells themselves have to be activated for performing its role in activation of other cells, including CTLs.
The role of TH-cells in activation of B-cells, as also it has been seen how TH-cells are themselves activated before they interact with B-cells (see Fig. 10.28 and Fig. 10.29). Let us now examine how activation of CTLs occurs with the help of TH-cells.
A pathogen entering into the body is first challenged by the phagocytic cells of innate defense system. These cells, like macrophages and dendritic cells ingest the pathogen and digest them with their hydrolytic enzymes. The resulting peptides derived from the proteins of the infecting microbe act as antigenic determinants and these are embedded in the matrix of the MHC Class I proteins and expressed on the surface of the phagocytes.
The phagocytes are now antigen-presenting cells (APC) and they migrate to the lymphoid tissues in search of appropriate CTLs with TCRs which match with the antigenic determinants present on their (APCs) surface. The CTLs are still in an inactive state. A CTL now recognizes the specific antigenic determinant present on an APC with the help of its TCR and binds to it. Such recognition is also helped by the CD8 protein which binds to MHC Class I protein of the APC. The binding of a CTL with an APC is the first step in activation of CTL.
ADVERTISEMENTS:
The APCs with antigenic determinants on MHC Class II proteins can recognize and interact with TH-cells carrying cognate TCRs. The interaction between an APC and a TH cell leads to their binding between the TCR and the antigenic determinant on one hand, and the CD4 protein of TH-cell and MHC protein of the APC on the other. This leads to stimulation of both the TH-cell and the APC and they produce a series of cytokines. The cytokine, interleukin-1, produced by the APC activates the TH– cell and the activated TH-cell produces interleukin-2 which induces the CTLs to proliferate and become killer cells.
The activated CTLs are now capable of attacking infected body cells. Such infected cells are recognized by the CTLs with the help of TCR which bind to the antigenic determinants expressed on the surface of body cells embedded in MHC Class I proteins. The CD8 protein of CTL binds to the MHC Class I protein of the infected body cells. The activation of TH-cells and CTLs is diagrammatically shown Fig. 10.43.
(v) Role of Cytotoxic T-Cells:
The cytotoxic T-cells play the key role in cell-mediated immunity. After activation, the CTLs become killer cells capable of destroying infected body cells, as well as malignant cells. These killer CTLs come out from the lymphoid tissues and enter into blood and lymph. While circulating they encounter the target cells and destroy them by the so-called ‘lethal hit’.
The activated CTLs have large granules in their cytoplasm. These contain the proteolytic enzymes, called granzymes, as also a toxic protein called perforin. The target cells which are mostly body cells infected by intracellular pathogens like viruses, some bacteria and occasionally some fungi, display the pathogen’s antigenic determinants on their surface complexed with MHC Class I proteins.
When a circulating killer CTL encounters an infected body cell, it recognizes the antigenic determinant and binds to it with its TCR and CD8 protein in a manner similar to its interaction with an APC. After a contact with an infected cell, the granules of the CTL migrate towards the point of contact and they release perforin molecules which polymerize to form a hole in the membrane of the target cell.
Through these pores, the granzymes enter into the cytoplasm of the target cell causing destruction of their contents. The target cell eventually undergoes lysis. This is called a ‘lethal hit’. The CTL can again synthesise the cytoplasmic granules and can attack another target cell.
ADVERTISEMENTS:
The cytotoxic effect of CTL is diagrammatically shown in Fig. 10.44:
In addition to infected body cells, activated CTLs can also inflict a lethal hit to cancer cells. These cells, like infected body cells, express specific tumour antigens complexed with MHC Class I on their surface and thus become target of CTL activity.
The circulating CTLs keep a strict watch on the appearance of such tumour cells and eliminate them promptly causing lysis. This is known as immune surveillance and it constitutes a very important phenomenon in keeping the body free of cancer cells which arise by transformation of normal body cells.
ADVERTISEMENTS:
Another important aspect of T-cell response is the production of a clone of memory T-cells (as shown in Fig. 10.43). Like memory B-cells, these cells remain dispersed throughout the body and search for the antigen which induced the initial T-cell response. The memory cells immediately pounce upon the antigen and destroy it. During this second encounter, the memory T-cells proliferates rapidly to induce a stronger secondary T-cell response.
In addition to direct killing of infected body cells and tumour cells, cytotoxic T-cells also produce different cytokines which are involved in defense against foreign invaders. Among these is a protein, called macrophage activating factor, which attracts macrophages to the site of infection and activates them. Another protein produced by the T-cells is the migration inhibitor which prevents macrophages to leave the infection site, so that the macrophages remain restricted to the site of infection.
The main function of the CTLs is on intracellular pathogens which are not attacked by the antibodies produced in humoral immunity. Through lethal hits resulting in lysis, such intracellular pathogen are exposed to the action of antibodies and they are eliminated through phagocytosis and neutralization. Thus, the cell-mediated immunity and humoral immunity work together to protect the body.