ADVERTISEMENTS:
In this article we will discuss about:- 1. Definition of Alveolar Air 2. Method of Collecting Alveolar Air 3. Composition 4. Partial Pressure of Gases 5. Tension of Gases 6. Method of Measurement 7. Effects 8. Factors 9. Alveolar PO2 and Venous Admixture.
Definition of Alveolar Air:
Alveolar air represents the air located in the respiratory part of the lungs which takes part in gas exchange with the blood in the pulmonary capillaries. Alveolar air, therefore, is a physiological quantity and does not represent the air located strictly in the anatomical alveoli.
It measures about 3000 ml and is the most important part of the air in the respiratory system since it is primarily responsible for oxygenation of venous blood and unloading the venous blood of adequate quantities of CO2. With a tidal volume of 500 ml about 350 ml of oxygen-rich atmospheric air mixes with the alveolar air to replenish the oxygen lost from alveolar air by absorption with the venous blood.
Method of Collecting Alveolar Air:
ADVERTISEMENTS:
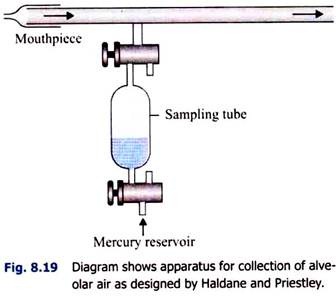
i. Haldane and Priestley’s Method (Fig. 8.19):
A tube, about 1.22 m (4 feet) long, is taken. It is fitted with a mouthpiece at one end. A side tube is attached very near to the mouthpiece and is attached to a sampling tube. Through the mouthpiece, the subject makes forcible expiration twice-at first (1) after normal inspiration and then (2) after normal expiration.
This is necessary because the alveolar air varies slightly in composition in different phases of respiration. Through the sampling tube, two samples of air are drawn in from the last part of the expired air, while the subject closes the mouthpiece with his tongue at the end of expiration.
ii. Otis-Rahn Method (Fig. 8.20):
ADVERTISEMENTS:
A second method of automatic collection of alveolar air has been described by Otis and Rahn method and is shown in the diagram (Fig. 8.20). During inspiration the end expiratory air of the previous expiration from the neighbourhood is drawn into the balloon by movement of the expiratory value.
During expiration the balloon is compressed so that no dead space air may contaminate the alveolar air collected into the balloon.
iii. Method of Analysis of Alveolar Air:
The classical method is to analyse alveolar air with Llyod’s modification of Haldane’s gas-analysis apparatus, which is used for analysis of respiratory gases. A measured quantity of air is admitted in the apparatus and its volume is accurately noted. The gas is then passed over caustic potash solution and its volume is noted again.
The diminution in volume is due to absorption of CO2 and from the difference between the original reading and the second reading the percentage of CO2 in the alveolar air may be calculated. The gas is now passed through alkaline pyrogallol solution which absorbs oxygen. From the reduction in volume the percentage of O2 in the alveolar air can be calculated.
Composition of Alveolar Air:
The composition of inspired air, normal expired air and alveolar air and also the tension of different gases are shown in the Table 8.2.
The increase in the percentage of nitrogen in the expired air is not real but relative. This is because the volume of the expired air is slightly less than that of inspired air, which is caused by the fact that the amount of CO2 evolved is less than the amount of oxygen absorbed. Consequently, in the percentage composition, nitrogen shows a relative rise.
ADVERTISEMENTS:
Further the composition of expired air varies depending on metabolic activity so that the composition of the alveolar air is kept as constant as possible.
Partial Pressure of Gases in Inspired Air Expired Air and Alveolar Air:
In a gas mixture, the pressure exerted by a particular gas is directly proportional to the percentage composition of the same gas in the mixture. Supposing in a mixture, oxygen constitutes 20% then oxygen will exert 20% (i.e., one fifth) of the total pressure. The Table 8.3 shows the partial pressures of the various gases.
It will be noted that the alveolar air differs in composition from that of the inspired (atmospheric) air.
ADVERTISEMENTS:
The reasons for this difference are:
i. Only a part of the alveolar air is replaced by inspired air .
ii. There is continuous absorption of O2 from the alveolar air by pulmonary venous blood – the alveolar air, therefore, is poorer in oxygen.
ADVERTISEMENTS:
iii. CO2 is added continuously to the alveolar air by the pulmonary venous blood- the alveolar air, therefore, is richer in CO2.
iv. The inspired air is dry but gets saturated with water vapour during its passage through the respiratory tract. Since some of the space in the alveoli is now occupied by water vapour, the space available for other gases is diminished.
Expired Air:
It has been noted that part of the expired air (‘dead space’ air) is atmospheric air rich in O2 and poor in CO2. As expiration progresses the expired air becomes a mixture of ‘dead space’ air and alveolar air and that the last part of the expired air is pure alveolar air. The expired air, therefore, is richer in O2 but poorer in CO2 as compared to alveolar air.
ADVERTISEMENTS:
Method of Collection of Expired Air:
Expired air is collected in a Douglas bag (Fig. 8.14) over a certain period of time. The sample of air is taken out from the side tube and analysed with the help of Llyod’s modification of Haldane gas analysis apparatus.
Tension of Gases in the Alveolar Air:
In the steady state the total tension of gases including water vapour in the alveoli is equal to the ambient barometric pressure. At sea-level the barometric pressure is 760 mmHg. The alveolar air is saturated with water vapour which exerts a tension of 47 mmHg at body temperature irrespective of the barometric pressure.
With these data in view it is possible to calculate tension of O2 and CO2 in the alveolar air provided the percentage composition of these gases in the alveolar air is known according to the ‘law of partial pressure’. Which states that the tension of a particular gas in a gas mixture is proportional to its percentage composition.
Thus in atmosphere the pressure of O2 is roughly 1/5th of 1 atmosphere = 153 mm Hg approximately. The atmospheric air, however, is dry. In calculation of tension of gases in the alveolar air, the pressure of water vapour (47 mm Hg) is to be deducted from the total pressure of gases in the alveoli which amounts to 760 mm Hg at sea-level.
Similarly tension of CO2 in the alveolar air is 5.5% of 713 = 39.2 mm Hg or 40 mm Hg (approx.). Of the 4 gases present in alveoli (O2 + CO2 + N2 + H2O vapour) the N2 and H2O vapour are neither taken into the blood stream nor added to alveolar air and as such the sum of partial pressure of these gases is constant in the alveoli.
This of course, means that the sum of partial pressure of O2 and CO2 is constant in the alveoli under steady state of metabolism and amounts roughly to 140 mm Hg at sea-level. A subject, therefore, can alter the PO2 of his alveolar air only at the expense of this alveolar PCO2 which is rather limited because the alveolar PCO2 cannot be reduced from normal 40 mm Hg to below 24 mm Hg.
Method of Measurement of Alveolar and Arterial PCO2:
Diffusion of CO2 is so rapid that alveolar PCO2 is always equal to arterial PCO2. Arterial PCO2 can be measured by direct electrochemical method using a CO2 sensitive electrode through an arterial puncture needle.
Indirect bloodless method has also been evolved by Campbell et, a and gives reliable result. An anaesthetic bag is filled with about 1 litre of O2 and the subject breaths and re-breaths into this bag for 1½ minutes. During this period there occurs an almost perfect ‘lung bag’ equilibrium so that the PCO2 of the bag air is almost equal to PCO2 of the alveolar air.
However to get an accurate result, the subject takes rest for at least 2 minutes and then re-breaths into the bag once again for 20 seconds during which period there occurs fine adjustment between the mixed venous PCO2 (PvCO2) and the bag air.
Recirculation of the blood and consequent higher PCO2 value is avoided by keeping the second rebreathing period limited to 20 seconds. After the end of the experiment the gas in the bag is analysed and its PCO2 is calculated. This is equal to mixed venous PCO2. The arterial PCO2 is always 6 mmHg lower than the venous PCO2.
Effects of Alveolar Air:
ADVERTISEMENTS:
i. Effect of Voluntary Hyperpnoea on Alveolar Air:
Hyperpnoea flushes out the alveoli with air so that the CO2 content and CO2 tension of the alveolar air and so of the arterial blood is diminished. This causes depression of respiration and may lead to temporary apnoea. During the depressed respiratory phase CO2 builds up again till the alveolar PCO2 attains its normal value and breathing is resumed. The alveolar PO2 rises in the early phase when the PCO2 is depressed but this has no effect on arterial saturation, which is normally 95% saturated.
ii. Effects of Voluntary Apnoea on Alveolar Air:
After normal inspiration breath can be held for 30 to 50 seconds. This is normal ‘breath-holding time’.
During breath -holding time the percentage of CO2 and PCO2 in the alveolar air gradually rises and the percentage of O2 and PO2 in the alveolar air gradually falls.
At the breaking point the O2 content of the alveolar air is about 8% (PO2=57 mm Hg) and its CO2 content is about 7% (PCO2 = 50 mm Hg). It will be noted that during apnoea there occurs a more significant fall in percentage and tension of oxygen in the alveolar air than rise of CO2 content and tension.
This is due to different shape of the dissociation curves of the two gases, so that removal of a given quantity of oxygen from blood will cause a more significant fall in oxygen tension than elevation of CO2 tension when the same volume of C2 is added.
Further CO2 being highly soluble most of it gets dissolved in body fluids and less is available for elevation of alveolar PCO2. Here the two stimuli of O2 lack and CO2 excess interact with each other in stimulation of respiration.
Inhalation of O2 before voluntary apnoea will prolong the breath-holding time by 15 to 20 seconds.
This is due to:
i. Absence of O2 lack stimulus during breath-holding period.
ii. The sensitivity of respiratory centre to CO2 excess is less in the absence of oxygen lack.
Voluntary hyper-apnoea before breath holding will wash off CO2 from the alveolar air and the breath- holding time will be increased considerably so that the subject may develop cyanosis due to O2 lack before the alveolar PCO2 rises sufficiently to stimulate the respiration. This shows that CO2 excess plays a more significant role in stimulating respiration than oxygen lack.
If a gas mixture with CO2 and O2 in quantities similar to that at the termination of the breath-holding time is re-breathed by a person at the time of breaking point he can hold his breath for a further period in spite of diminished oxygen and elevated CO2 in the lungs. This indicates other factors besides O2 lack and CO2 excess play an important role in precipitation respiratory movement.
During the period of apnoea a larger volume of O2 is removed than the volume of CO2 added to the lungs. This causes a sense of discomfort and distress. Further, holding the chest in a fixed position stimulates afferents from the respiratory muscles which are obviated by the re-breathing experiment cited above. The afferent stimuli originating in the chest muscles, therefore, constitute the third important factor in determining the breaking point after breath-holding.
iii. Effects of High Altitude on Alveolar Oxygen:
At high altitude the barometric pressure falls and so the tension of gases in the inspired air and also in the alveolar air falls. Water vapour exerts a tension of 47 mmHg at all altitudes and CO2 is continuously exerted from the body into the respiratory alveoli. As a result of this adverse combination, the PO2 of the alveolar air falls at high altitude. Further the fall of alveolar PO2 is disproportionately low because of extremely low O2 tension of tissues, O2 is absorbed very quickly from the alveoli.
The formula for calculating alveolar PO2 at different altitudes is:
Alveolar PO2 = PB – PCO2 – 47 / 5 – PO2 loss (PO2 loss is the oxygen pressure decrease caused by oxygen uptake into the blood and the value 47 is the vapour pressure of water.)
There occurs a light fall in alveolar PCO2 due to hyperventilation but this cannot compensate for O2 deficit at high altitude. The Table 8.4 gives alveolar PCO2 and PO2 at different altitudes along with barometric pressure (PB) and percentage saturation of arterial blood with oxygen.
ADVERTISEMENTS:
It may be noted that an altitude of 15 km or 50,000 feet the barometric pressure is only 87 mmHg. This therefore, is the total pressure of gases and water vapour in the alveoli, the latter accounts for 47 mm Hg leading only (87 – 47) = 40 mm Hg for O2, N2 and CO2. Since PCO2 is 24 mm Hg, only 16 mm Hg of pressure is distributed between N2 and O2 present in the ratio of approximately 4:1.
That being so on theoretical ground, one would expect that the alveolar PO2 at this altitude would be about 3 mm Hg. But due to increased uptake of O2 by grossly anoxic tissues at this altitude the alveolar PO2 falls to 1 mm Hg only. Even if pure O2 is inhaled and all the N2 in the alveoli is replaced by it – the PO2 in alveoli would be increased to 16 mm Hg only. Oxygen, therefore, must be administered under pressure to sustain life at this altitude.
Factors Controlling Alveolar PCO2:
Two important factors are:
1. Metabolic Rate:
Metabolic rate, i.e., production of CO2 in the body. The higher metabolic rate the greater will be the PACO2.
2. Alveolar Ventilation:
The greater the alveolar ventilation the lower will be the PACO2 and the higher will be PAO2.
Alveolar PO2 and Venous Admixture:
There is normally a difference between pulmonary capillary PO2 (Pco2) and arterial PO2. Alveolar PO2 which normally is in near equilibrium with pulmonary end-capillary PO2 therefore, is higher than arterial PO2.
The difference is explained by the fact that under normal conditions some blood passes from the venous side to the arterial side avoiding the lungs and without being exposed to alveolar O2. For example, the Thebesian veins draining directly into the left heart, the bronchpulmonary anastomosis already mentioned.
It has been estimated that about 6% of venous blood pumped by the right heart by-passes the lungs altogether and thus reduces the PO2 of the pulmonary venous blood. This is called shunt effect and by definition it is anatomical shunt-contributing to venous admixture.
Physiological Shunt:
Even under physiological conditions the millions of alveoli of the lungs are not adequately ventilated and some of them may be completely non-ventilated. Blood flowing through the non-ventilated alveoli will not be oxygenated at all and so will contribute to venous admixture effect. Similarly venous blood flowing through poorly ventilated alveoli will not be adequately oxygenated and thus will produce the same effect as noted above.
It is possible that both hypoventilated and hyperventilated alveoli may be present in the same lung but because of the fact that normally ventilated alveoli can effect almost complete saturation of haemoglobin with oxygen – the defective oxygenation of venous blood perfused through hypoventilated alveoli cannot be compensated by perfusion of hyperventilated alveoli. From quantitative aspect, therefore, physiological shunt is larger than anatomical shunt. It is the commonest cause of hypoxia in respiratory diseases.
The concept of anatomical and physiological shunts is analogous to the concept of anatomical and physiological dead space. Normally alveolar ventilation is about 4 litres/minute and about 5 litres of blood (cardiac output) is perfused through the alveoli per minute. The average ventilation/perfusion ratio for the whole lungs, therefore, is 4/5 or 0.8.
Increase in physiological dead space is due to absence or diminution of blood flow through well-ventilated alveoli – the condition, therefore, is attended with increase in ventilation/perfusion ratio. The reverse is true in increase in ‘shunt’ or venous admixture effect when the ratio of ventilation to perfusion is decreased.