ADVERTISEMENTS:
This article throws light upon physical and genetic mapping of genome. The three things to know about are:
(1) Genetic Techniques Used for Cross-Breeding Experiment (2) Molecular Markers in Physical Mapping and (3) Restriction Mapping of DNA Fragments.
Thing # 1. Genetic Techniques Used for Cross-Breeding Experiment:
Genetic mapping is based on the use of genetic techniques to construct maps showing the positions of genes and other sequences features on a genome. These genetic techniques include cross-breeding experiments or, in the case of humans, the examination of family histories.
ADVERTISEMENTS:
Genetic mapping is based on the principles of inheritance as first described by Gregor Mendel in 1865 and genetic linkages.
Genetic maps are created to locate the genes or characters on the chromosome for their utilization in genetic studies. Physical maps are created to identify certain markers to detect or diagnose the specific character.
i. Genetic Linkage:
Genetic linkage occurs when particular genetic loci or alleles for genes are inherited jointly. Genetic linkage was first discovered by the British geneticists William Bateson and Reginald Punnett shortly after Mendel’s laws were rediscovered. Genetic loci on the same chromosome are physically connected and tend to stay together during meiosis, and are thus genetically linked. For example, in fruit flies the genes affecting eye color and wing length are inherited together because they appear on the same chromosome.
Alleles for genes on different chromosomes are usually not linked, due to independent assortment of chromosomes during meiosis. Because there is some crossing over of DNA when the chromosomes segregate, alleles on the same chromosome can be separated and go to different daughter cells. There is a greater probability of this happening if the alleles are far apart on the chromosome, as it is more likely that a cross-over will occur between them. The relative distance between two genes can be calculated using the offspring of an organism showing two linked genetic traits, and finding the percentage of the offspring where the two traits do not run together.
ADVERTISEMENTS:
The higher the percentage of descendants that does not show both traits, the further apart on the chromosome they are. Among individuals of an experimental population or species, some phenotypes or traits occur randomly with respect to one another in a manner known as independent assortment.
Today scientists understand that independent assortment occurs when the genes affecting the phenotypes are found on different chromosomes or separated by a great enough distance on the same chromosome that recombination occurs at least half of the time. But in many cases, even genes on the same chromosome that are inherited together produce offspring with unexpected allele combinations. These results from a process called crossing over.
At the beginning of normal meiosis, a chromosome pair (made up of a chromosome from the mother and a chromosome from the father) intertwine and exchange sections or fragments of chromosome. The pair then breaks apart to form two chromosomes with a new combination of genes that differs from the combination supplied by the parents. Through this process of recombining genes, organisms can produce offspring with new combinations of maternal and paternal traits that may contribute to or enhance survival.
ii. Genetic Map:
A genetic map is a linkage map of a species or experimental population that shows the position of its known genes and/or genetic markers relative to each other in terms of recombination frequency during crossover of homologous chromosomes. The greater the frequency of recombination (segregation) between two genetic markers, the farther apart they are assumed to be. Conversely, the lower the frequency of recombination between the markers, the smaller the physical distance between them.
Historically, the markers originally used were detectable phenotypes (enzyme production, color, shapes etc.) derived from coding DNA sequences. Now, non-coding DNA sequences such as microsatellites or those generating restriction fragment length polymorphisms (RFLPs) have been used. Genetic maps help researchers to locate other markers, such as other genes by testing for genetic linkage of the already known markers. A genetic map is not a physical map or gene map.
To be useful in genetic analysis, a gene must exist in at least two forms, or alleles; each specifying a different phenotype. Earlier only those genes could be studied whose specifying phenotypes were distinguishable by visual observation. This approach soon became outdated as in many cases a single phenotypic character could be affected by more than one gene. For example, in 1922, 50 genes had been mapped onto the four fruit fly chromosomes, but nine of these genes were for eye color.
The observations by Thomas Hunt Morgan that the amount of crossing over between linked genes differs (partial linkage) led to the idea that crossover frequency might indicate the distance separating genes on the chromosome. Morgan’s student Alfred Sturtevant developed the first genetic map, also called a linkage map.
iii. Recombination Frequency:
Sturtevant assumed that crossing over was a random event, there being an equal chance of it occurring at any position along a pair of lined-up chromatids. He proposed that the greater the distance between linked genes, the greater the chance that non-sister chromatids would cross over in the region between the genes. By working out the number of recombinants it is possible to obtain a measure for the distance between the genes. This distance is called a genetic map unit (m.u.), or a centimorgan and is defined as the distance between genes for which one product of meiosis in 100 is recombinant.
A recombinant frequency (RF) of 1% is equivalent to 1 m.u. A linkage map is created by finding the map distances between a numbers of traits that are present on the same chromosome, ideally avoiding having significant gaps between traits to avoid the inaccuracies that will occur due to the possibility of multiple recombination events.
ADVERTISEMENTS:
Recombination frequency is the frequency that a chromosomal crossover will take place between two loci (or genes) during meiosis. Recombination frequency is a measure of genetic linkage and is used in the creation of a genetic linkage map. During meiosis, chromosomes assort randomly into gametes, such that the segregation of alleles of one gene is independent of alleles of another gene. This is stated in Mendel’s second law and is known as the law of independent assortment.
The law of independent assortment always holds true for genes that are located on different chromosomes, but for genes that are on the same chromosome, it does not always hold true. As an example of independent assortment, consider the crossing of the pure-bred homozygote parental strain with genotype AABB with a different pure-bred strain with genotype aabb. A and a and B and b represent the alleles of genes A and B. Crossing these homozygous parental strains will result in F1 generation offspring with genotype AaBb.
The F1 offspring AaBb produces gametes that are AB, Ab, aB, and ab with equal frequencies (25%) because the alleles of gene A assort independently of the alleles for gene B during meiosis. Note that 2 of the 4 gametes (50 %) Ab and aB-were not present in the parental generation. These gametes represent recombinant gametes. Recombinant gametes are those gametes that differ from both of the haploid gametes that made up the diploid cell. In this example, the recombination frequency is 50% since 2 of the 4 gametes were recombinant gametes.
The recombination frequency will be 50% when two genes are located on different chromosomes or when they are widely separated on the same chromosome. This is a consequence of independent assortment. When two genes are close together on the same chromosome, they do not assort independently and are said to be linked. Linked genes have a recombination frequency that is less than 50%.
ADVERTISEMENTS:
As an example of linkage, consider the classic experiment by William Bateson and Reginald Punnett. They were interested in trait inheritance in the sweet pea and were studying two genes- the gene for flower color (P- purple and p- red) and the gene affecting the shape of pollen grains (L- long and I- round). They crossed the pure lines PPLL and ppll and then self-crossed the resulting PpLl lines.
According to Mendelian genetics, the expected phenotypes would occur in a 9:3:3:1 ratio of PL:P1:pL:p1. To their surprise, they observed an increased frequency of PL and pi and a decreased frequency of P1 and pL (Table 21.1).
Their experiment revealed linkage between the P and L alleles and the p and l alleles. The frequency of P occurring together with L and with p occurring together with I is greater than that of the recombinant PI and pL. The recombination frequency cannot be computed directly from this experiment, but it is less than 50%. The progeny in this case received two dominant alleles linked on one chromosome (referred to as coupling or cis arrangement).
ADVERTISEMENTS:
However, after crossover, some progeny could have received one parental chromosome with a dominant allele for one trait (e.g., Purple) linked to a recessive allele for a second trait (eg round) with the opposite being true for the other parental chromosome (e.g., red and long). This is referred to as repulsion or a Trans-arrangement.
The phenotype here would still be purple and long but a test cross of this individual with the recessive parent would produce progeny with much greater proportion of the two crossover phenotypes. While such a problem may not seem likely from this example, unfavorable repulsion linkages do appear while breeding for disease resistance in some crops.
When two genes are located on the same chromosome, the chance of a crossover producing recombination between the genes is directly related to the distance between the two genes. Thus, the use of recombination frequencies has been used to develop linkage maps or genetic maps.
iv. Genetic Mapping in Bacteria:
Bacteria are haploid organisms and do not undergo meiosis. So for creating their genetic maps geneticists made use of other methods to induce crossovers between homologous segments of bacterial DNA.
ADVERTISEMENTS:
They used the three methods of recombination that occurs in bacteria:
(a) Conjugation-Two bacteria come into physical contact and one bacterium (the donor) transfers DNA to the second bacterium (the recipient). The transferred DNA can be a copy of some or possibly the donor cell’s entire chromosome, or it could be a segment of chromosome DNA up to 1 mb in length integrated in a plasmid. The latter is called episome transfer,
(b) Transduction- It involves transfer of a small segment of DNA up to 50 kb or so, from donor to recipient via a bacteriophage,
(c) Transformation- The recipient cell takes up from its environment a fragment of DNA, rarely longer than 50 kb, released from a donor cell.
In bacteria, the phenotype studied are the biochemical characteristics like ability to synthesize tryptophan in the dominant or wild type strain and inability to synthesize tryptophan in other strain, which is the recessive allele. The gene transfer is usually set up between a donor strains that possesses dominant gene to the recipient strain that possesses recessive gene. The transfer into the recipient is monitored by looking for attainment of the biochemical function specified by the gene being studied. This can be understood by (Fig. 21.1). Here, the functional gene for tryptophan synthesis from a wild strain is being transferred to recipient that lacks the functional copy of that gene (trp–).
This recipient is called as auxotroph (bacteria which can survive only if provided with tryptophan). The wild strain (trp+) does not require tryptophan for its survival. After the transfer, two crossovers are needed to integrate the transferred gene into the recipient cell’s chromosome, converting the recipient from trp” to trp+.
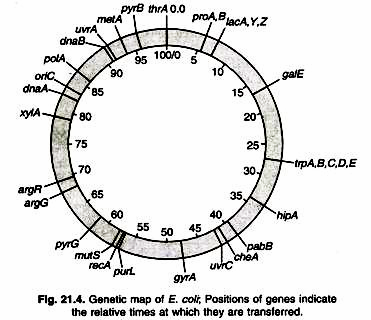
The precise detail of the map depends on the method of gene transfer being used. During conjugation, DNA is transferred from donor to recipient in the same way that a string is pulled through a tube. The relative positions of markers on the DNA molecule can therefore be mapped by determining the times at which the markers appear in the recipient cell. For example in Fig. 21.2, markers A, B and C are transferred after 8, 20 and 30 minutes of beginning of conjugation. The entire E. coli DNA takes approx. 100 minutes to transfer.
In case of transformation and transformation mapping enable genes that are relatively close together to be mapped, because the transferred DNA segment is short (<50kb), so the probability of two genes being transferred together depends on how close together they are on the bacterial DNA (Fig. 21.3).
Elie Wollman and Francois Jacob (1950s) conducted first genetic mapping experiments in bacteria. They studied linear transfer of genes in conjugation experiments between Hfr (Hfr- High frequency of recombination) and F– (F– fertility factor) strains of E. coli. During the experiment they interrupted conjugation between bacteria at specific times termed as “Interrupted mating”. They noticed that the time it takes for genes to enter a recipient cell is directly related to their order along the chromosome.
This experiment derived them to give the hypothesis that (a) The chromosome of the Hfr donor is transferred in a linear manner to the F- recipient cell (b) The order of genes along the chromosome can be deduced by determining the time required for various genes to enter the recipient.
ADVERTISEMENTS:
Conjugation studies have been used to map over 1,000 genes along the circular E. coli chromosome. The genetic maps are scaled in minutes e.g., E. coli chromosome is 100 minutes long, conjugative transfer of the complete chromosome takes approximately 100 minutes (Fig. 21.4).
v. Gene Mapping in Humans by Pedigree Analysis:
To map human chromosomes, obviously one cannot perform controlled mating experiments. However, it is possible to estimate map positions by examining linkage in several generations of relatives. This means that only limited data are available, and their interpretations is often difficult because a human marriage rarely results in a convenient test cross, and often the genotypes of one or more family members are unobtainable because those individuals are dead or unwilling to cooperate.
For example, blood samples from several large Mormon families in Utah, where all the members of at least three generations were alive to be sampled, have been collected and stored. These have already been used to establish genetic linkage relationships and will be available in the years ahead to study other human genes as they are identified.
Thing # 2. Molecular Markers in Physical Mapping:
Different types of molecular markers are used to understand and ascertain relationship in different organisms/individuals as well as to detect or diagnose character. These markers are to locate certain characteristics on the gel (banding pattern) which can be used to detect a specific character/defect in the genome. Unlike genetic mapping, physical mapping is not to locate the genes/characters on a genome, but to create a unique pattern by processing the genomic DNA.
There are several molecular markers available which are used depending upon the objective of the work and facilities available at the centre. Use of these markers to create maps (e.g., electrophoretic patterns) of an organism is known as ‘physical mapping’. Molecular markers used in physical mapping are described below. New technologies are also developed simultaneously to resolve biological problems and help legal proceedings.
Restriction Fragment Length Polymorphism (RFLP):
RFLP is a method used by molecular biologists to follow a particular sequence of DNA as it is passed on to other cells. RFLPs can be used in many different settings to accomplish different objectives. RFLPs can be used in paternity cases or criminal cases to determine the source of a DNA sample.
RFLPs can be used to determine the disease status of an individual. RFLPs can be used to measure recombination rates which can lead to a genetic map with the distance between RFLP loci measured in centiMorgans.
RFLP, as a molecular marker, is specific to a single clone/restriction enzyme combination. It is a difference in homologous DNA sequences that can be detected by the presence of fragments of different lengths after digestion of the DNA samples in question with specific restriction endonucleases. Most RFLP markers are co-dominant (both alleles in heterozygous sample will be detected) and highly locus-specific.
An RFLP probe is a labelled DNA sequence that hybridizes with one or more fragments of the digested DNA sample after they were separated by gel electrophoresis, thus revealing a unique blotting pattern characteristic to a specific genotype at a specific locus. Short, single- or low-copy genomic DNA or cDNA clones are typically used as RFLP probes. The RFLP probes are frequently used in genome mapping and in variation analysis (genotyping, forensics, paternity tests, hereditary disease diagnostics, etc.) (Fig. 21.5).
i. Procedure:
Usually, DNA from an individual specimen is first extracted and purified. Purified DNA may be amplified by polymerase chain reaction (PCR), The DNA is then cut into restriction fragments using suitable endonucleases, which only cut the DNA molecule where there are specific DNA sequences, termed recognition sequence or restriction sites that are recognized by the enzymes.
These sequences are specific to each enzyme, and may be either four, six, eight, ten or twelve base pairs in length. The more base pairs there are in the restriction site, the more specific it is and the lower the probability that it will find a place to be cut. The restriction fragments are then separated according to length by agarose gel electrophoresis. The resulting gel may be enhanced by Southern blotting. Alternatively, fragments may be visualized by pre-treatment or post-treatment of the agarose gel, using methods such as ethidium bromide staining or silver staining respectively.
RFLPs have provided valuable information in many areas of biology, including: screening human DNA for the presence of potentially deleterious genes (Fig. 21.6). Providing evidence to establish the innocence of or a probability of the guilt of, a crime suspect by DNA “fingerprinting”. The distance between the locations cut by restriction enzymes (the restriction sites) varies between individuals, due to insertions, deletions or trans-versions.
This causes the length of the fragments to vary, and the position of certain amplicons differs between individuals (thus polymorphism). This can be used to genetically tell individuals apart. It can also show the genetic relationship between individuals, because children inherit genetic elements from their parents. Mitochondrial DNA RFLP analyses can lead to the determination of maternal relationships.
Fragments may also be used to determine relationships among and between species by comparison of the resulting haplotypes (abridged for ‘haploid genotype’). RFLP is a technique used in marker assisted selection. Terminal Restriction Fragment Length Polymorphism (TRFLP or sometimes T-RFLP) is a molecular biology technique initially developed for characterizing bacterial communities in mixed-species samples. The technique has also been applied to other groups including soil fungi.
The technique works by PCR amplification of DNA using primer pairs that have been labelled with fluorescent tags. The PCR products are then digested using RFLP enzymes and the resulting patterns visualized using a DNA sequencer. The results are analyzed either by simply counting and comparing bands or peaks in the TRFLP profile, or by matching bands from one or more TRFLP runs to a database of known species.
ii. Measurement of distance between two RFLP loci:
To calculate the genetic distance between two loci, you need to be able to observe recombination. Traditionally, this was performed by observing phenotypes but with RFLP analysis, it is possible to measure the genetic distance between two RFLP loci whether they are a part of genes or not. Let’s look at a simple example in fruit flies. Two RFLP loci with two RFLP bands possible at each locus (Fig. 21.7).
These loci are located on the same chromosome for the female (left) and the male (right). The upper locus can produce two different bands called 1 and 3. The lower locus can produce bands called 2 or 4. The male is homozygous for band 1 at the upper locus and 2 for the lower locus. The female is heterozygous at both loci. Their RFLP banding patterns can be seen on the Southern blot below (Fig. 21.8).
The male can only produce one type of gamete (1 and 2) but the female can produce four different gametes. Two of the possible four are called parental because they carry both RFLP bands from the same chromosome; 1 and 2 from the left chromosome or 3 and 4 from the right chromosome. The other two chromosomes are recombinant because recombination has occurred between the two loci and thus the RFLP bands are mixed so that 1 is now linked to 4 and 3 is linked to 2.
When these two flies mate, the frequency of the four possible progeny can be measured and from this information, the genetic distance between the two RFLP loci (upper and lower) can be determined (Fig. 21.9).
In this example, 70% of the progeny were produce from parental genotype eggs and 30% were produced by recombinant genotype eggs. Therefore, these two RFLP loci are 30 centiMorgans apart from each other.
iii. PCR-RFLP:
Isolation of sufficient DNA for RFLP analysis is time consuming and labor intensive. However, PCR can be used to amplify very small amounts of DNA, usually in 2-3 hours, to the levels required for RFLP analysis. Therefore, more samples can be analyzed in a shorter time. An alternative name for the technique is Cleaved Amplified Polymorphic Sequence (CAPS) assay.
iv. Limitations:
RFLP is a multistep procedure involving restriction enzymatic cleavage, electrophoresis, southern blotting and detection of specific sequences. It is a time consuming process.
Random Amplified Polymorphic DNA (RAPD):
This technique can be used to determine taxonomic identity, assess kinship relationships, detect inter-specific gene flow, analyze hybrid speciation, and create specific probes. Advantages of RAPDs include suitability for work on anonymous genomes, applicability to work where limited DNA is available, efficiency and low expense. It is also useful in distinguishing individuals, cultivars or accessions. RAPDs also have applications in the identification of asexually reproduced plant varieties for forensic or agricultural purposes, as well as ecological ones.
In RAPD by using different primers, molecular characters can be generated that are diagnostic at different taxonomic levels. This is really a stripped-down version of PCR but uses a single sequence in the design of the primer (i.e., two primers are still needed for PCR: the same primer is used at either end).
The primer may be designed specifically, but could be chosen randomly and is used to amplify a series of samples which will include both the material of interest as well as other control samples with which the experimental material needs to be compared. Choice of primer length will be critical to the determination of band complexity in the resulting amplification pattern. Eventually a particular probe will be found that is able to distinguish between the sample of interest and those that are different.
i. Procedure:
Unlike traditional PCR analysis, RAPD (pronounced ‘rapid’) does not require any specific knowledge of the DNA sequence of the target organism: the identical 10-mer primers will or will not amplify a segment of DNA, depending on positions that are complementary to the primers’ sequence. For example, no fragment is produced if primers annealed too far apart or 3′ ends of the primers are not facing each other.
Therefore, if a mutation has occurred in the template DNA at the site that was previously complementary to the primer, a PCR product will not be produced, resulting in a different pattern of amplified DNA segments on the gel (Fig. 21.10). RAPD is an inexpensive yet powerful typing method for many bacterial species
RAPD amplification products can be either variable (polymorphic) or constant (non- polymorphic). In a RAPD analysis of several individuals within a species, and species within a genus, constant fragments diagnostic for a genus may be identified, as well as fragments which are polymorphic between species of the genus. RAPDs can be applied to analyze fusion of genotypes at different taxonomic levels. At the level of the individual, RAPD markers can be applied to parentage analysis, while at the population level, RAPD can detect hybrid populations, species or subspecies.
The detection of genotype hybrids relies on the identification of diagnostic RAPD markers for the parental genotypes under investigation. However RAPD markers tend to underestimate genetic distances between distantly related individuals, for example in inter-specific comparisons.
It is wise to be cautious when using RAPD for taxonomic studies above the species level. Conventional RFLP techniques are ill-suited for the analysis of paternity and estimation of reproductive success in species with large offspring clutches, because of the need to determine paternity for each individual offspring. RAPD fingerprinting provides a ready alternative for such cases.
Synthetic offspring may be produced by mixing equal amounts of the DNA of the mother and the potential father. The amplification products from the synthetic offspring should ideally contain the full complement of bands that appear in any single offspring of these parents (Table 21.2).
ii. Limitations of RAPD:
1. Nearly all RAPD markers are dominant, i.e., it is not possible to distinguish whether a DNA segment is amplified from a locus that is heterozygous (1 copy) or homozygous (2 copies). Co-dominant RAPD markers, observed as different-sized DNA segments amplified from the same locus, are detected only rarely.
2. PCR is an enzymatic reaction, therefore the quality and concentration of template DNA, concentrations of PCR components, and the PCR cycling conditions may greatly influence the outcome. Thus, the RAPD technique is notoriously laboratory dependent and needs carefully developed laboratory protocols to be reproducible.
3. Mismatches between the primer and the template may result in the total absence of PCR product as well as in a merely decreased amount of the product. Thus, the RAPD results can be difficult to interpret.
Amplification Fragment Length Polymorphism (AFLP):
Amplified Fragment Length Polymorphism (AFLP) is a polymerase chain reaction (PCR) based genetic fingerprinting technique that was developed in the early 1990’s by Keygene. AFLP can be used in the fingerprinting of genomic DNA of varying origins and complexities. The amplification reaction is rigorous, versatile and robust, and appears to be quantitative.
While AFLP is capable of producing very complex fingerprints (100 bands where RAPD produces 20), it is a technique that requires DNA of reasonable quality and is more experimentally demanding. AFLP uses restriction enzymes to cut genomic DNA, followed by ligation of complementary double stranded adaptors to the ends of the restriction fragments.
A subset of the restriction fragments are then amplified using 2 primers complementary to the adaptor and restriction site fragments. The fragments are visualized on denaturing polyacrylamide gels either through auto-radiographic or fluorescence methodologies.
i. Procedure:
AFLP-PCR is a highly sensitive method for detecting polymorphisms in DNA. The technique was originally described by Vos and Zabeau in 1993. The procedure of this technique is divided into three steps (Fig. 21.11):
1. Digestion of total cellular DNA with one or more restriction enzymes that cuts frequently (Msel, 4 bp recognition sequences) and one that cuts less frequently (EcoRI, 6 bp recognition sequence). The resulting fragments are ligated to end-specific adaptor molecules.
2. Selective amplification of some of these fragments with two PCR primers that have corresponding adaptor and restriction site specific sequences.
3. Electrophoretic separation of amplicons on a gel matrix, followed by visualisation of the band pattern.
In a second, “selective”, PCR, using the products of the first as template, primers containing two further additional bases, chosen by the user, are used. The EcoRI-adaptor specific primer used bears a label (fluorescent or radioactive). Gel electro-phoretic analysis reveals a pattern (fingerprint) of fragments representing about 1/4000th of the EcoRl-Msel fragments.
AFLP’s, can be co-dominant markers, like RFLP’s. Co-dominance results when the polymorphism is due to sequences within the amplified region. Yet, because of the number of bands seen at one time, additional evidence is needed to establish that a set of bands result from different alleles at the same locus.
If, however, the polymorphism is due to presence/absence of a priming site, the relationship is dominance. The non-priming allele will not be detected as a band. Compared to RAPD, fewer primers should be needed to screen all possible sites. AFLP can be used for mapping, fingerprinting and genetic distance calculation between genotypes. The advantage of AFLP is its high multiplexity and therefore the possibility of generating high marker densities.
One limitation of the AFLP technique is that fingerprints may share few common fragments when genome sequence homology is less than 90%. Therefore, AFLP cannot be used in comparative genomic analysis with hybridization-based probes or when comparing genomes that are evolving rapidly such as those of some microbes. Conversely, very homogeneous genomes may not be suitable for AFLP analysis.
A study on the genetic diversity of an endangered alpine plant (Eryngium alpinum L. (Apiaceae) demonstrated that AFLP markers enable a quick and reliable assessment of intraspecific genetic variability in conservation genetics. The study showed that although the endangered plant occurred in small isolated populations, these populations contained a high genetic diversity, a good indication that recovery of the species was possible.
ii. Limitations of AFLP:
1. Proprietary technology is needed to score heterozygotes and homozygotes. Otherwise, AFLP must be dominantly scored.
2. Developing locus-specific markers from individual fragments can be difficult.
3. Need to use different kits adapted to the size of the genome being analyzed.
Microsatellites:
Microsatellites can be used to determine genetic diversity within a species, as well as being able to distinguish varieties and even individuals, as well as parentage. The distribution of genetic variability is commonly used to verify species, subspecies or population division. Monitoring change in diversity may also be useful for predicting populations in peril as the persistence of a population partially depends on maintaining its evolutionary significance which requires genetic variation.
Microsatellites have been used to estimate demographic bottlenecks in some species. A bottleneck, when it severely and temporarily reduces population size, can also drastically reduce the genetic diversity of a population. A common theme in conservation genetics is the use of genetic variation to identify populations that have experienced bottlenecks, as numerous threatened or endangered species and populations have been found to have low levels of genetic variation.
Inter-Simple Sequence Repeats (ISSR):
ISSRs can be used to assess hybridization in natural populations of plants, as a study on Penstemon (Scrophulariaceae) did. Eight ISSR primers were used to examine patterns of hybridization and hybrid speciation in a hybrid complex involving four species, as well as allowing examination of pollen-mediated gene flow. Previous studies using allozymes, restriction-site variation of nuclear rDNA and chloroplast DNA failed to determine whether gene flow occurs among species other than P. cenranthifolius.
The previous studies also failed to provide support for hypotheses of diploid hybrid speciation. ISSR proved to be a much more successful technique in this study, allowing all species and all DNA accessions to be differentiated. ISSR has also been used to detect varieties and diversity in rice, revealing much more data than RFLPs. The technique allowed for dissection below the subspecies level and this gives it a good level of applicability in the study of rare or endangered plants.
ISSRs have been used in conjunction with RAPD data to determine the colonization history of Olea europaea in Macronesia, along with lineages in the species complex. The two techniques have also been utilized in examining the historical biogeography of Sea rocket (Cakile maritima) and Sea Holly (Eryngium maritimum), comparing different and only distantly related taxa of broadly similar extant distribution. The trees generated by the different methods were largely similar topologically. Using the result, dispersal routes of the species along a linear coast line could be construed.
Joint use of RAPD and ISSR has also been used to examine clonal diversity in Calamagrostis porteri ssp. insperata (Poaceae), a rare grass that has little or no sexual reproduction, and spreads by vegetative reproduction. The relative advantages and disadvantages of various molecular markers in physical mapping are summarized in Table 21.3. This information suggests that RFLP, SSR and AFLP markers are most effective in detecting polymorphism.
However, given the large amount of DNA required for RFLP detection and the difficulties in automating RFLP analysis, AFLP and SSR are currently most popular markers.
The main uses of these markers include:
1. Assessment of genetic variability and characterization of germplasm.
2. Identification and fingerprinting of genotypes.
3. Estimation of genetic distances between population, inbreeds and breeding material.
4. Detection of monogenic and qualitative trait loci.
5. Marker assisted selection.
6. Identification of sequences of useful candidate genes.
Thing # 3. Restriction Mapping of DNA Fragments:
Restriction Mapping:
Genetic mapping using RFLPs as DNA markers can locate the positions of polymorphic restriction sites within a genome, but very few of the restriction sites in a genome are polymorphic, so many sites are not mapped by this technique.
We increase the marker density on a genome map by using an alternative method to locate the positions of some of the non polymorphic restriction sites. This is what restriction mapping achieves, although in practice the technique has limitations that means it is applicable only to relatively small DNA molecules.
Methodology for Restriction Mapping:
The simplest way to construct a restriction map is to compare the fragment sizes produced when a DNA molecule is digested with two different restriction enzymes that recognize different target sequences. An example using the restriction enzymes EcoRI and BamHI is shown in figure. 21.12. First, the DNA molecule is digested with just one of the enzymes and the sizes of the resulting fragments measured by agarose gel electrophoresis. Next, the molecule is digested with the second enzyme and the resulting fragments again sized in an agarose gel.
The results of subsequent use of two enzymes give clear picture about restriction sites creating a large number of fragments but this method do not allow their relative positions to be determined. Additional information is therefore obtained by cutting the DNA molecule with both enzymes together. In the example shown in Figure 21.12, the double restriction enables three of the sites to be mapped. However, a problem arises with the larger EcoRI fragment because this contains two BamHI sites and there are two alternative possibilities for the map location of the outer one of these.
The problem dissolved by going back to the original DNA molecule and treating it again with BamHI on its own, but this time preventing the digestion from going to completion by, for example, incubating the reaction for only a short time rousing a suboptimal incubation temperature. This is called a partial restriction and leads to a more complex set of products. The complete restriction products now being supplemented with partially restricted fragments that still contain one or more uncut BamHI sites.
In the example shown in Figure 21.12, the size of one of the partial restriction fragments is diagnostic and the correct map can be identified. A partial restriction usually gives the information needed to complete a map, but if there are many restriction sites then this type of analysis becomes bulky, simply because there are many different fragments to consider. An alternative strategy is simpler because it enables the majority of the fragments to be ignored. This is achieved by attaching a radioactive or other type of marker to each end of the starting DNA molecule before carrying out the partial digestion.
The result is that many of the partial restriction products become “invisible” because they do not contain an end-fragment and so do not show up when the agarose gel is screened for labeled products (Fig. 21.12). The sizes of the partial restriction products that are visible enable unmapped sites to be positioned relative to the ends of the starting molecule.
The scale of restriction mapping is limited by the sizes of the restriction fragments. Restriction maps are easy to generate if there are relatively few cut sites for the enzymes being used. However, as the number of cut sites increases, so also do the numbers of single, double and partial-restriction products whose sizes must be determined and compared in order for the map to be constructed. Computer analysis can be brought into play but problems still eventually arise.
A stage will be reached when a digest contains so many fragments that individual bands merge on the agarose gel, increasing the chances of one or more fragments being measured incorrectly or missed out entirely. If several fragments have similar sizes then even if they can all be identified, it may not be possible to assemble them into a clear map. Restriction mapping is therefore more applicable to small rather than large molecules, with the upper limit for the technique depending on the frequency of the restriction sites in the molecule being mapped.
In practice, if a DNA molecule is less than 50 kb in length it is usually possible to construct a clear restriction map for a selection of enzymes with six nucleotide recognition sequences. Restriction maps are equally useful after bacterial or eukaryotic genomic DNA has been cloned, if the cloned fragments are less than 50kb in length, because a detailed restriction map can then be built up as a preliminary to sequencing the cloned region. This is an important application of restriction mapping in projects sequencing large genomes.
Restriction mapping can be used for mapping of entire genomes larger than 50kb by slightly eliminating the limitations of restriction mapping by choosing enzymes expected to have infrequent cut sites in the target DNA molecule.
These “rare cutters” fall into two categories:
1. A few restriction enzymes cut at seven- or eight-nucleotide recognition sequences. Examples are Sapl (5′-GCTCTTC-3′) and SgfI (5′-GCGATCGC-3′). The enzymes with seven-nucleotide recognition sequences would be expected, on average, to cut a DNA molecule with GC content of 50% once every 47 = 16,384 bp.
The enzymes with eight nucleotide recognition sequences should cut once every 48 = 65,536 bp. These figures compare with 46 = 4096 bp for enzymes with six-nucleotide recognition sequences, such as BamHI and EcoRI.
Cutters with seven-or eight-nucleotide recognition sequences are often used in restriction mapping of large molecules, but the approach is not as useful as it might by simply because not many of these enzymes are known.
2. Enzymes can be used whose recognition sequences contain motifs that are rare in the target DNA. Genomic DNA molecules do not have random sequences and some molecules are significantly deficient in certain motifs. For example, the sequence 5′-CG- 3′ is rare in the genomes of vertebrates because vertebrate cells possess an enzyme that adds a methyl group to carbon 5 of the C nucleotide in this sequence.
Domination of the resulting 5-methylcytosine gives thymine. The consequence is that during vertebrate evolution, many of the 5′-CG’3 sequences that were originally in these genomes have become converted to 5′-TG-3′.
Restriction enzymes that recognize a site containing 5′- CG-3′ therefore cut vertebrate DNA relatively infrequently. Examples are Smal (5′-CCCGGG-3′), which cuts human DNA once every 78 kb on average, and BssHII (5′- GCGCGC’3′), which cuts once every 390 kb.
The potential of restriction mapping is therefore increased by using rare cutters. It is still not possible to construct restriction maps of the genomes of animals and plants, but it is feasible to use the technique with large cloned fragments, and with the smaller DNA molecules of prokaryotes and lower eukaryotes such as yeast and fungi.
If a rare cutter is used then it may be necessary to employ a special type of agarose gel electrophoresis to study the resulting restriction fragments. This is because the relationship between the length of DNA molecule and its migration rate in an electrophoresis gel is not linear, the resolution decreasing as the molecules get longer (Fig. 21.13A). This means that it is not possible to separate molecules more than about 50 kb in length, because all of these longer molecules run as a single, slowly migrating band in a standard agarose gel.
To separate them it is necessary to replace the linear electric field used in conventional gel electrophoresis with a more complex field. An example is provided by orthogonal field alternation gel electrophoresis (OFAGE), in which the electric field alternates between two pairs of electrodes, each positioned at an angle of 45″ to the length of the gel (Fig. 21.13B).
The DNA molecules still move down through the gel, but each change in the field forces the molecules to realign. Shorter molecules realign more quickly than longer ones and so migrate more rapidly through the gel. The overall result is that molecules much longer than those separated by conventional gel electrophoresis can be resolved. Related techniques include CHEF (contour clamped homogeneous electric fields) and FIGE (field inversion gel electrophoresis).