ADVERTISEMENTS:
The marker genes are of two types:
I. Selectable marker genes.
II. Reporter genes.
Type # I. Selectable Marker Genes:
The selectable marker genes are usually an integral part of plant transformation system. They are present in the vector along with the target gene. In a majority of cases, the selection is based on the survival of the transformed cells when grown on a medium containing a toxic substance (antibiotic, herbicide, antimetabolite). This is due to the fact that the selectable marker gene confers resistance to toxicity in the transformed cells, while the non- transformed cells get killed.
ADVERTISEMENTS:
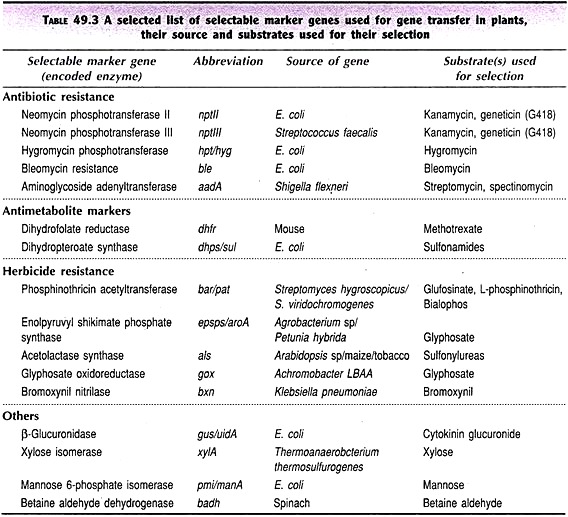
A large number of selectable marker genes are available and they are grouped into three categories— antibiotic resistance genes, antimetabolite marker genes, and herbicide resistance genes (Table 49.3).
(a) Antibiotic Resistance Genes:
In many plant transformation systems, antibiotic resistance genes (particularly of E. coli) are used as selectable markers. Despite the plants being eukaryotic in nature, antibiotics can effectively inhibit the protein biosynthesis in the cellular organelles, particularly in chloroplasts. Some of the antibiotic resistance selectable marker genes are briefly described.
ADVERTISEMENTS:
Neomycin phosphotransferase II (npt II gene):
The most widely used selectable marker is npt II gene encoding the enzyme neomycin phosphotransferase II (NPT II). This marker gene confers resistance to the antibiotic kanamycin. The trans-formants and the plants derived from them can be checked by applying kanamycin solution and the resistant progeny can be selected.
Hygromycin phosphotransferase (hpt gene):
The antibiotic hygromycin is more toxic than neomycin and therefore can kill non-transformed plant cells much faster. Hygromycin phosphotransferase (hpt) gene thus provides resistance to transformed cells.
Aminoglycoside adenyltransferase (aadA gene):
Aminoglycoside 3′-adenyltransferase (aadA) gene confers resistance to transformed plant cells against the antibiotics streptomycin and spectionomycin.
(b) Antimetabolite Marker Genes:
Dihydrofolate reductase (dhfr gene):
The enzyme dihydrofolate reductase, produced by dhfr gene is inhibited by the antimetabolite methotrexate. A mutant dhfr gene in mouse that codes for this enzyme which has a low affinity to methotrexate has been identified. This dhfr gene fused with CaMV promoter results in a methotrexate resistant marker which can be used for the selection of transformed plants.
(c) Herbicide Resistance Markers:
ADVERTISEMENTS:
Genes that confer resistance to herbicides are in use as markers for the selection of transgenic plants.
Phosphinothricin acetytransferase (pat/bar gene):
Bialophos, phosphinothricin and glufosinate are commonly used herbicides. The pat/bar genes code for phosphinothricin acetyltransferase which converts these herbicides into acetylated forms that are non-herbicidal. Thus, pat/bar genes confer resistance to the transformed plants.
Enolpyruvylshikimate phosphate synthase (epsps/aroA genes):
ADVERTISEMENTS:
The herbicide glyphosate inhibits photosynthesis. It blocks the activity of enolpyruvylshikimate phosphate (EPSP) synthase, a key enzyme involved in the biosynthesis of phenylalanine, tyrosine and tryptophan. Mutant strains of Agrobacterium and Petunia hybrida that are resistant to glyphosate have been identified. The genes epsps/aroA confer resistance to transgenic plants which can be selected.
Bromoxynil nitrilase (bxn gene):
The herbicide bromoxynil inhibits photosynthesis (photosystem II). Bromoxynil nitrilase enzyme coded by the gene bxn inactivates this herbicide. The gene bxn can be successfully used as a selectable marker for the selection of transformed plants.
Production of Marker-Free Transgenic Plants:
There is a growing concern among the public regarding the use of antibiotic or herbicide resistance genes as selectable markers of plant transformation:
ADVERTISEMENTS:
i. The products of some marker genes may be toxic or allergic.
ii. The antibiotic resistance might be transferred to pathogenic microorganisms in the soil.
iii. There is a possibility of creation of super weeds that are resistant to normally used herbicides.
iv. A transgenic plant with selectable marker genes cannot be transformed again by using the same selectable markers.
ADVERTISEMENTS:
In light of the apprehensions listed above, the public is concerned about the safety of transgenic technology, particularly related to the selectable marker genes (antibiotic/herbicide resistance genes). There are fears about the safety of consumption of foodstuffs derived from genetically engineered plants. This is despite the fact that so far none of the marker genes have been shown to adversely affect human, animal or environmental safety.
Clean Gene Technology:
The process of developing transgenic plants without the presence of selectable marker genes or by use of more acceptable marker genes is regarded as clean gene technology. And this will result in the production of many marker-free transgenic plants that will be readily acceptable by the public. Some of the approaches for clean gene technology are given.
Avoiding selectable marker genes:
Theoretically, it is possible to totally avoid marker genes and introduce only the transgene of interest. The transformed paints can then be screened by an advanced technique like polymerase chain reaction and the desirable plants selected. This approach is not practicable due to cost factor.
Co-transformation with two DNAs:
The transgenic plants can be produced by employing two separate DNAs — one carrying the desired target gene and the other the marker gene. The transformed plants contain both the genes, but at different sites on the chromosomal DNA. Traditional breeding techniques (a few rounds) can be used to get rid of the transgenic plants with selectable markers.
ADVERTISEMENTS:
Removal of selectable markers:
It is possible to selectively remove the selectable marker genes from the plant genome. For this purpose, site-specific recombinase systems are utilized. Several recombinase systems are in fact available which can be used to selectively excise the marker genes from the plant genome.
Cloning of selectable markers between transposable elements:
A selectable marker gene can be cloned between plant transposable elements (Ds elements) and then inserted. The selectable marker is planked by the sequences that increase the intra-chromosomal recombination. This results in the excision of the marker gene.
Type # II. Reporter Genes:
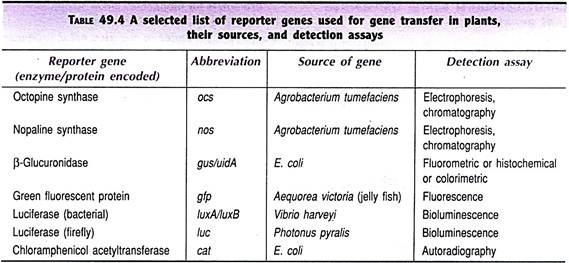
A reporter gene may be regarded as the test gene whose expression can be quantified. The plant transformation can be assessed by the expression of reporter genes (also called as screenable or scoreable genes). In general, an assay for the reporter gene is carried out by estimating the quantity of the protein it produces or the final products formed. A selected list of the reporter genes along with the detection assays is given in Table 49.4, some of the important ones are discussed below.
Opine synthase (ocs, nos genes):
ADVERTISEMENTS:
The common opines present in T-DNA of Ti or Ri plasmids of Agrobacterium are octopine and nopaline, respectively produced by the synthase genes ocs and nos. The transformed status of the plant cells can be easily detected by the presence of these opines. Opines can be separated by electrophoresis and identified. Alternately, the enzyme activities responsible for the production of opines can also be assayed.
β-Glucuronidase (gusluidA gene):
ADVERTISEMENTS:
β-Glucuronidase producing gene (gusluidA) is the most commonly used reporter gene in assessing plant transformation for the following reasons:
i. β-Glucuronidase assays are very sensitive.
ii. Quantitative estimation of the enzyme can be done by fluorometric method (using substrate 4-methylumbelliferryl P-D-glucuronide which is hydrolysed to 4-methylumbelliferone).
iii. Qualitative data on the enzyme can be obtained by histochemical means (enzyme localization can be detected by chromogenic substance such as substrate X-gluc).
iv. No need to extract and identify DNA.
Green fluorescent protein (gfp gene):
Green fluorescent protein (GFP), coded by gfp gene, is being widely used in recent years. In fact, in many instances, GFP has replaced GUS since assays of GFP are easier and non-destructive. Thus, screening of even the primary transplants can be done by GFP which is not possible with other reporter genes.
Gene for GFP has been isolated from jelly fish Aequorea victoria which is a luminescent organism. The original gfp gene has been significantly modified to make it more useful as a reporter gene. GFP emits fluorescence which can be detected under a fluorescent microscope.
Bacterial luciferase (luxA/luxB genes):
The bacterial luciferase genes (luxA and luxB) have originated from Vibrio harveyi. They can be detected in some plant transformation vectors. The detection assay of the enzyme is based on the principle of bioluminescence. Bacterial luciferase catalyses the oxidation of long-chain fatty aldehydes that results in the emission of light which can be measured.
Firefly luciferase (luc gene):
The enzyme firefly luciferase, encoded by the gene luc, catalyses the oxidation of D-luciferin (ATP dependent) which results in the emission of light that can be detected by sensitive luminometers. The firefly luciferase gene, however, is not widely used as a marker gene since the assay of the enzyme is rather cumbersome.
Chloramphenicol acetyl transferase (cat gene):
The cat gene producing chloramphenicol acetyl transferase (CAT) is a widely used reporter gene in mammalian cells. Due to the availability of GUS and GFP reporter systems for plant trans-formants, CAT is not commonly used. However, some workers continue to use CAT by a sensitive radioactive assay, for the detection of the reporter gene cat.