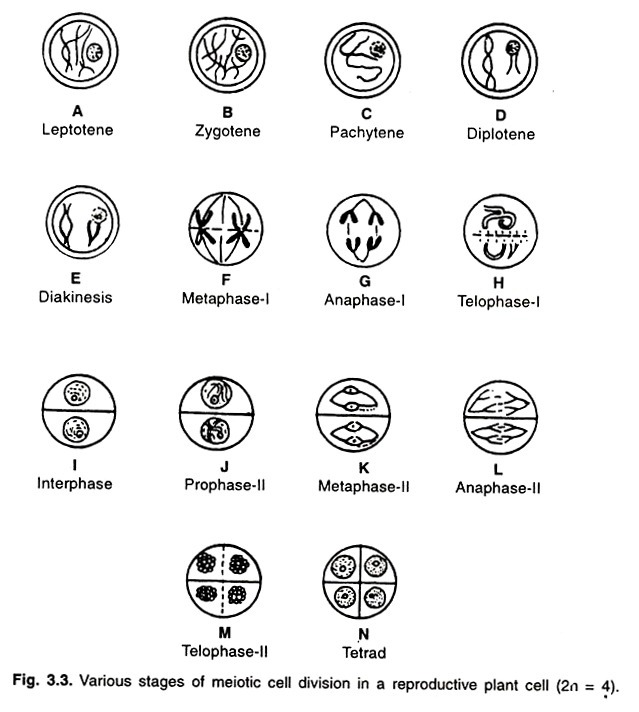
ADVERTISEMENTS:
This article throws light upon the two types of gene therapy. The two types are: (1) Ex Vivo Gene Therapy and (2) In Vivo Gene Therapy.
There are two types of gene therapies:
I. Ex vivo gene therapy:
ADVERTISEMENTS:
This involves the transfer of genes in cultured cells (e.g., bone marrow cells) which are then reintroduced into the patient.
II. In vivo gene therapy:
The direct delivery of genes into the cells of a particular tissue is referred to as in vivo gene therapy.
Type # I. Ex Vivo Gene Therapy:
The ex vivo gene therapy can be applied to only selected tissues (e.g., bone marrow) whose cells can be cultured in the laboratory. The technique of ex vivo gene therapy involves the following steps (Fig. 13.2).
1. Isolate cells with genetic defect from a patient.
2. Grow the cells in culture.
3. Introduce the therapeutic gene to correct gene defect.
4. Select the genetically corrected cells (stable trans-formants) and grow.
5. Transplant the modified cells to the patient.
The procedure basically involves the use of the patient’s own cells for culture and genetic correction, and then their return back to the patient. This technique is therefore, not associated with adverse immunological responses after transplanting the cells. Ex vivo gene therapy is efficient only, if the therapeutic gene (remedial gene) is stably incorporated and continuously expressed. This can be achieved by use of vectors.
Vectors in Gene Therapy:
The carrier particles or molecules used to deliver genes to somatic cells are referred to as vectors. The important vectors employed in ex vivo gene therapy are listed below and briefly described next.
i. Viruses
ii. Human artificial chromosome
ADVERTISEMENTS:
iii. Bone marrow cells.
i. Viruses:
The vectors frequently used in gene therapy are viruses, particularly retroviruses. RNA is the genetic material in retroviruses. As the retrovirus enters the host cell, it synthesizes DNA from RNA (by reverse transcription). The so formed viral DNA (referred to as provirus) gets incorporated into the DNA of the host cell.
The proviruses are normally harmless. However, there is a tremendous risk, since some of the retroviruses can convert normal cells into cancerous ones. Therefore, it is absolutely essential to ensure that such a thing does not happen.
ADVERTISEMENTS:
Making retroviruses harmless:
Researchers employ certain biochemical methods to convert harmful retroviruses to harmless ones, before using them as vectors. For instance, by artificially removing a gene that encodes for the viral envelope, the retrovirus can be crippled and made harmless. This is because, without the envelope, retrovirus cannot enter the host cell. The production of a large number (billions) of viral particles can be achieved, starting from a single envelope defective retrovirus (Fig. 13.3).
This is made possible by using helper viruses which contain normal gene for envelope formation. Along with the helper virus, the vector (with defective envelope gene) can enter the host cell and both of them multiply. By repeated multiplication in host cells, billions of vector and helper viruses are produced.
ADVERTISEMENTS:
The vector viruses can be separated from the helper viruses and purified. Isolation of vector viruses, totally free from helper viruses, is absolutely essential. Contamination of helper viruses is a big threat to the health of the patients undergoing gene therapy.
Retroviruses in gene therapy:
The genetic map of a typical retrovirus is depicted in Fig. 13.4A. In general, the retrovirus particle has RNA as a genome organized into six regions. It has a 5′-long terminal repeat (5′-LTR), a non-coding sequence required for packaging RNA designated as psi (Ψ), a gene gag coding for structural protein, a gene pol that codes for reverse transcriptase, a gene env coding for envelope protein and a 3-LTR sequence.
For use of a retrovirus as a vector, the structural genes gag and pol are deleted. These genes are actually adjacent to Ψ region. In addition, a promoter gene is also included (Fig. 13.4B). This vector design allows the synthesis of cloned genes. A retroviral vector can carry a therapeutic DNA of maximum size of 8 kb.
ADVERTISEMENTS:
A retroviral vector DNA can be used to transform the cells. However, the efficiency of delivery and integration of therapeutic DNA are very low. In recent years, techniques have been developed to deliver the vector RNA to host cells at a high frequency. For this purposes, packaged retroviral RNA particles are used. This technique allows a high efficiency of integration of pharmaceutical DNA into host genome.
Several modified viral vectors have been developed in recent years for gene therapy. These include oncoretrovirus, adenovirus, adenoassociated virus, herpes virus and a number of hybrid vectors combining the good characters of the parental vectors.
Murine leukemia viruses in gene therapy:
ADVERTISEMENTS:
This is a retrovirus that causes a type of leukemia in mice. It can react with human cells as well as the mouse cells, due to a similarity in the surface receptor protein. Murine leukemia virus (MLV) is frequently used in gene transfer.
AIDS virus in gene therapy?
It is suggested that the human immunodeficiency virus (HIV) can be used as a vector in gene transfer. But this is bound to create public uproar. Some workers have been successful in creating a harmless HIV (crippled HIV) by removing all the genes related to reproduction. At the same time, the essential genes required for gene transfer are retained. There is a distinct advantage with HIV when compared with MLV. MLV is capable of bringing out gene transfer only in dividing cells. HIV can infect even non-dividing cells (e.g., brain cells) and do the job of gene transfer effectively. However, it is doubtful whether HIV can ever be used as a vector.
ii. Human Artificial Chromosome:
The details of human artificial chromosome (HAC) are described elsewhere .HAC is a synthetic chromosome that can replicate with other chromosomes, besides encoding a human protein. As already discussed above, use of retroviruses as vectors in gene therapy is associated with a heavy risk. This problem can be overcome if HAC is used. Some success has been achieved in this direction.
iii. Bone Marrow Cells:
ADVERTISEMENTS:
Bone marrow contains totipotent embryonic stem (ES) cells. These cells are capable of dividing and differentiating into various cell types (e.g., red blood cells, platelets, macrophages, osteoclasts, B- and T-lymphocytes). For this reason, bone marrow transplantation is the most widely used technique for several genetic diseases.
And there is every reason to believe that the genetic disorders that respond to bone marrow transplantation are likely to respond to ex vivo gene therapy also (Table 13.2). For instance, if there is a gene mutation that interferes with the function of erythrocytes (e.g., sickle-cell anemia), bone marrow transplantation is done. Bone marrow cells are the potential candidates for gene therapy of sickle-cell anemia. However, this is not as simple as theoretically stated.
Selected Examples of Ex Vivo Gene Therapy:
Therapy for Adenosine Deaminase Deficiency:
The first and the most publicized human gene therapy was carried out to correct the deficiency of the enzyme adenosine deaminase (ADA). This was done on September 14, 1990 by a team of workers led by Blaese and Anderson at the National Institute of Health, USA (The girl’s name is Ashanti, 4 years old then).
Severe combined immunodeficiency (SCID):
This is rare inherited immune disorder associated with T-lymphocytes, and (to a lesser extent) B-lymphocytes dysfunction. About 50% of SCID patients have a defect in the gene (located on chromosome 20, and has 32,000 base pairs and 12 exons) that encodes for adenosine deaminase. In the deficiency of ADA, deoxyadenosine and its metabolites (primarily deoxyadenosine 5′-triphosphate) accumulate and destroy T-lymphocytes.
T-Lymphocytes are essential for body’s immunity. Besides participating directly in body’s defense, they promote the function of B-lymphocytes to produce antibodies. Thus, the patients of SCID (lacking ADA) suffer from infectious diseases and die at an young age. Previously, the children suffering from SCID were treated with conjugated bovine ADA, or by bone marrow transplantation.
Technique of therapy for ADA deficiency:
The general scheme of gene therapy adopted for introducing a defective gene in the patient has been depicted in Fig 13.2. The same procedure with suitable modifications can also be applied for other gene therapies.
A plasmid vector bearing a pro-viral DNA is selected. A part of the pro-viral DNA is replaced by the ADA gene and a gene (G 418) coding for antibiotic resistance, and then cloned. The antibiotic resistance gene will help to select the desired clones with ADA gene.
A diagrammatic representation of the treatment of ADP deficient patient is depicted in Fig. 13.5.
Circulating lymphocytes are removed from a patient suffering from ADA deficiency. These cells are transfected with ADA gene by exposing to billions of retroviruses carrying the said gene. The genetically-modified lymphocytes are grown in cultures to confirm the expression of ADA gene and returned to the patient. These lymphocytes persist in the circulation and synthesize ADA.
Consequently, the ability of the patient to produce antibodies is increased. However, there is a limitation. The lymphocytes have a short life span (just live for a few months), hence the transfusions have to be carried out frequently.
Transfer of ADA gene into stem cells:
In 1995, ADA gene was transferred into the stem cells, obtained from the umbilical cord blood, at the time of baby’s delivery. Four days after birth, the infant received the modified cells back. By this way, a permanent population of ADA gene producing cells was established.
Therapy for Familial Hypercholesterolemia:
The patients of familial hypercholesterolemia lack the low density lipoprotein (LDL) receptors on their liver cells. As a result, LDL cholesterol is not metabolised in liver. The accumulated LDL- cholesterol builds up in the circulation, leading to arterial blockage and heart diseases.
Attempts are being made by gene therapists to help the victims of familial hypercholesterolemia. In fact, there is some success also. In a woman, 15% of the liver was removed. The hepatocytes were transduced with retroviruses carrying genes for LDL receptors. These genetically modified hepatocytes were infused into the patient’s liver.
The hepatocytes established themselves in the liver and produced functional LDL-receptors. A significant improvement in the patient’s condition, as assessed by estimating the lipid parameters in blood, was observed. Further, there were no antibodies produced against the LDL-receptor molecules, clearly showing that the genetically modified liver cells were accepted.
Therapy for Lesch-Nyhan Syndrome:
Lesch-Nyhan syndrome is an inborn error in purine metabolism due to a defect in a gene that encodes for the enzyme hypoxanthine-guanine phosphoribosyl transferase (HCPRT). In the absence of HGPRT, purine metabolism is disturbed and uric acid level builds up, resulting in severe gout and kidney damage. The victims of Lesch- Nyhan syndrome exhibit symptoms of mental retardation, besides an urge to bite lips and fingers, causing self-mutilation.
By using retroviral vector system, HGPRT producing genes were successfully inserted into cultured human bone marrow cells. The major problem in humans is the involvement of brain. Experiments conducted in animals are encouraging. However, it is doubtful whether good success can be achieved by gene therapy for Lesch-Nyhan syndrome in humans, in the near future.
Therapy for Hemophilia:
Hemophilia is a genetic disease due lack of a gene that encodes for clotting factor IX. It is characterized by excessive bleeding. By using a retroviral vector system, genes for the synthesis of factor IX were inserted into the liver cells of dogs. These dogs no longer displayed the symptoms of hemophilia.
Ex Vivo Gene Therapy with Non-Autologous Cells:
The ex vivo gene therapies described above are based on the transplantation of genetically modified cells for the production of desired proteins. However, there are several limitations in using the patient’s own cells (autologous cells) for gene therapy. These include lack of enough cells from target tissues, defective uptake of genes and their inadequate expression. To overcome these problems, attempts are on to develop methods to use non-autologous cells (i.e., cells from other individuals or animals). The outline of the procedure is briefly described below.
Tissue-specific cells capable of growing in culture are selected. These include fibroblasts from skin, hepatocytes from liver, and myoblasts from muscle and astrocytes from brain. These cells are cultured and genetically modified with the therapeutic gene. They are then encapsulated in artificial membrane composed of a synthetic polymer (e.g., polyether sultone, alginase-poly L-lysine-alginate).
The polymeric membranes are non-immunogenic, therefore the patient can accept non-autologous encapsulated cells. Further, being semipermeable in nature, these membranes allow the nutrients to enter in, and the encoded protein (by the therapeutic gene) to pass out.
Experiments conducted in animals have shown some encouraging results for using non-autologous cells in gene therapy. The encapsulated cells were found to proliferate and produce the required protein. However, the success has been very limited in human trials.
Type # II. In Vivo Gene Therapy:
The direct delivery of the therapeutic gene (DNA) into the target cells of a particular tissue of a patient constitutes in vivo gene therapy (Fig. 13.6). Many tissues are the potential candidates for this approach. These include liver, muscle, skin, spleen, lung, brain and blood cells. Gene delivery can be carried out by viral or non- viral vector systems. The success of in vivo gene therapy mostly depends on the following parameters
i. The efficiency of the uptake of the remedial (therapeutic) gene by the target cells.
ii. Intracellular degradation of the gene and its uptake by nucleus.
iii. The expression capability of the gene.
In vivo gene therapy with special reference to gene delivery systems (viral, non-viral) with suitable examples is described.
Gene Delivery by Viruses:
Many viral vector systems have been developed for gene delivery. These include retroviruses, adenoviruses, adenoassociated viruses and herpes simplex virus.
Retrovirus vector system:
Replication defective retrovirus vectors that are harmless are being used. A plasmid in association with a retrovirus, a therapeutic gene and a promoter is referred to as plasmovirus. The plasmovirus is capable of carrying a DNA (therapeutic gene) of size less than 3.4 kb. Replication defective virus particles can be produced from the plasmovirus.
As such, for the delivery of genes by retroviral vectors, the target cells must be in a dividing stage. But majority of the body cells are quiescent. In recent years, viral vectors have been engineered to infect non-dividing cells. Further, attempts are on to include a DNA in the retroviral vectors (by engineering env gene) that encodes for cell receptor protein. If this is successfully achieved, the retroviral vector will specifically infect the target tissues.
Adenoviral vector system:
Adenoviruses (with a DNA genome) are considered to be good vectors for gene delivery because they can infect most of the non-dividing human cells. A common cold adenovirus is a frequently used vector. As the target cells are infected with a recombinant adenovirus, the therapeutic gene (DNA) enters the nucleus and expresses itself.
However, this DNA does not integrate into the host genome. Consequently, adenoviral based gene therapy required periodic administration of recombinant viruses. The efficiency of gene delivery by adenoviruses can be enhanced by developing a virus that can specifically infect target cells. This is possible by incorporating a DNA encoding a cell receptor protein.
Adeno-associated virus vector system:
Adeno-associated virus is a human virus that can integrate into chromosome 19. It is a single-stranded, non-pathogenic small DNA virus (4.7 kb). As the adeno-associated virus enters the host cell, the DNA becomes double- stranded, gets integrated into chromosome and expresses.
Adeno-associated viruses can serve as good vectors for the delivery of therapeutic genes. Recombinant viruses are created by using two plasmids and an adenovirus (i.e., helper virus) by a special technique. Some attempts were made to use therapeutic genes for the treatment of the human diseases-hemophilia (for production of blood clotting factor IX) and cystic fibrosis (for synthesis of cystic fibrosis trans membrane regulator protein) by employing adeno-associated viruses.
Therapy for cystic fibrosis:
Cystic fibrosis (CF) is one of the most common (frequency 1: 2,500) and fatal genetic diseases. It is characterized by the accumulation of sticky, dehydrated mucus in the respiratory tract and lungs. Patients of CF are highly susceptible to bacterial infections in their lungs and most of them die before reaching the age of thirty. Cystic fibrosis can be traced in European folklore, the following statement used to be said “Woe to that child which when kissed on the forehead tastes salty. He is be witched and soon must die”.
Biochemical basis:
In the normal persons the chloride ions of the cells are pushed out through the participation of a protein called cystic fibrosis trans membrane regulator (CFTR). In the patients of cystic fibrosis, the CFTR protein is not produced due to a gene defect. Consequently, the chloride ions concentrate within the cells which draw water from the surroundings. As a result, the respiratory tract and the lungs become dehydrated with sicky mucus, an ideal environment for bacterial infections.
Gene therapy:
As the defective gene for cystic fibrosis was identified in 1989, researchers immediately started working on gene therapy for this disease. Adenoviral vector systems have been used, although the success has been limited. The major drawback is that the benefits are short-lived, since the adenoviruses do not integrate themselves into host cells. Multiple administration of recombinant adenovirus caused immunological responses that destroyed the cells.
By using adeno-associated virus vector system, some encouraging results were reported in the gene therapy of CF. In the phase I clinical trials with CF patients, the vector persisted for about 70 days and some improvement was observed in the patients. Some researchers are trying to insert CF gene into the developing fetal cells (in experimental animals such as mice) to produce CFTR protein. But a major breakthrough is yet to come.
Herpes simplex virus vector system:
The retroviruses and adenoviruses employed in in vivo gene therapy are engineered to infect specific target cells. There are some viruses which have a natural tendency to infect a particular type of cells. The best example is herpes simplex virus (HSV) type I, which infects and persists in non-dividing nerve cells. HSV is a human pathogen that causes (though rarely) cold sores and encephalitis.
These are a large number of diseases (metabolic, neurodegenerative, immunological, tumors) associated with nervous system. HSV is considered as an ideal vector for in vivo gene therapy of many nervous disorders.
The HSV has a double-stranded DNA of about 152 kb length as its genome. About 30 kb of HSV genome can be replaced by a cloned DNA without loss of its basic characteristics (replication, infection, packaging etc.). But there are some technical difficulties in dealing with large-sized DNAs in genetic engineering experiments. Some modified HSV vectors with reduced genomic sizes have been developed.
Most of the work on the gene therapy, related to the use of HSV as a vector, is being conducted in experimental animals. And the results are quite encouraging. HSV vectors could deliver therapeutic genes to the brain and other parts of nervous system. These genes are well expressed and maintained for long periods. More research, however, is needed before going for human trials. If successful, HSV may help to treat many neurodegenerative syndromes such as Parkinson’s disease and Alzheimer’s disease by gene therapy.
Gene Delivery by Non-Viral Systems:
There are certain limitations in using viral vectors in gene therapy. In addition to the prohibitive cost of maintaining the viruses, the viral proteins often induce inflammatory responses in the host. Therefore, there is a continuous search by researchers to find alternatives to viral vector systems.
Pure DNA constructs:
The direct introduction of pure DNA constructs into the target tissue is quite simple. However, the efficiency of DNA uptake by the cells and its expression are rather low. Consequently, large quantities of DNA have to be injected periodically. The therapeutic genes produce the proteins in the target cells which enter the circulation and often get degraded.
Lipoplexes:
The lipid-DNA complexes are referred to as lipoplexes or more commonly liposomes. They have a DNA construct surrounded by artificial lipid layers. A large number of lipoplexes have been prepared and used. They are non-toxic and non-immunogenic.
The major limitation with the use of lipoplexes is that as the DNA is taken up by the cells, most of it gets degraded by the lysosomes. Thus, the efficiency of gene delivery by lipoplex is very low. Some clinical trials using liposome-CFTR gene complex showed that the gene expression was very short-lived.
DNA-molecular conjugates:
The use of DNA-molecular conjugates avoids the lysosomal breakdown of DNA. Another advantage of using conjugates is that large-sized therapeutic DNAs (> 10 kb) can be delivered to the target tissues. The most commonly used synthetic conjugate is poly-L-lysine, bound to a specific target cell receptor. The therapeutic DNA is then made to combine with the conjugate to form a complex (Fig. 13.7).
This DNA molecular conjugate binds to specific cell receptor on the target cells. It is engulfed by the cell membrane to form an endosome which protects the DNA from being degraded. The DNA released from the endosome enters the nucleus where the therapeutic gene is expressed.
Human artificial chromosome:
Human artificial chromosome (HAC) which can carry a large DNA one or more therapeutic genes with regulatory elements is a good and ideal vector. Studies conducted in cell cultures using HAC are encouraging. But the major problem is the delivery of the large-sized chromosome into the target cells. Researchers are working to produce cells containing genetically engineered HAC. There exists a possibility of encapsulating and implanting these cells in the target tissue. But a long way to go!
Efficiency of gene delivery by non-viral vectors:
Although the efforts are continuously on to find suitable non-viral vectors for gene delivery, the success has been very limited. This is mainly due to the following two reasons.
1. The efficiency of transfection is very low.
2. The expression of the therapeutic gene is for a very short period, consequently there is no effective treatment of the disease.
Gene Therapy Strategies for Cancer:
Cancer is the leading cause of death throughout the world, despite the intensive treatment strategies (surgery, chemotherapy, radiation therapy). Gene therapy is the latest and a new approach for cancer treatment. Some of the developments are briefly described hereunder.
Tumor necrosis factor gene therapy:
Tumor necrosis factor (TNF) is a protein produced by human macrophages. TNF provides defense against cancer cells. This is brought out by enhancing the cancer-fighting ability of tumor- infiltrating lymphocytes (TILs), a special type of immune cells.
The tumor-infiltrating lymphocytes were transformed with a TNF gene (along with a neomycin resistant gene) and used for the treatment of malignant melanoma (a cancer of melanin producing cells usually occurs in skin). TNF as such is highly toxic, and fortunately no toxic side effects were detected in the melanoma patients injected with genetically altered TILs with TNF gene. Some improvement in the cancer patients was observed.
Suicide gene therapy:
The gene encoding the enzyme thymidine kinase is often referred to as suicide gene, and is used for the treatment of certain cancers. Thymidine kinase (TK) phosphorylates nucleosides to form nucleotides which are used for the synthesis of DNA during cell division. The drug ganciclovir (GCV) bears a close structural resemblance to certain nucleosides (thymidine). By mistake, TK phosphorylates ganciclovir to form triphosphate-GCV, a false and unsuitable nucleotide for DNA synthesis. Triphosphate-GCV inhibits DMA polymerase (Fig. 13.8).
The result is that the elongation of the DNA molecule abruptly stops at a point containing the false nucleotide (of ganciclovir). Further, the triphosphate-GCV can enter and kill the neighbouring cancer cells, a phenomenon referred to as bystander effect. The ultimate result is that the cancer cells cannot multiply, and therefore die. Thus, the drug ganciclovir can be used to kill the cancer cells.
Ganciclovir is frequently referred to as a pro-drug and this type of approach is called pro-drug activation gene therapy. Ganciclovir has been used for treatment of brain tumors (e.g., glioblastoma, a cancer of glial cells in brain), although with a limited success.
In the suicide gene therapy, the vector used is herpes simplex virus (HSV) with a gene for thymidine kinase (TK) inserted in its genome. Normal brain cells do not divide while the brain tumor cells go on dividing unchecked.
Thus, there is a continuous DNA replication in tumor cells. By using GCV-HSVTK suicide gene therapy, some reduction in proliferating tumor cells was reported. Several new strategies are being developed to increase the delivery of HSVTK gene to all the cells throughout a tumor.
ADVERTISEMENTS:
Two-gene cancer therapy:
For treatment of certain cancers, two gene systems are put together and used. For instance, TK suicide gene (i.e., GCV-HSVTK) is clubbed with interleukin-2 gene (i.e. a gene promoting immunotherapy). Interleukin-2 produced mobilizes immune response. It is believed that certain proteins are released from the tumor cells on their death.
These proteins, in association with immune cells, reach the tumor and initiate immunological reactions directed against the cancer cells. Two-gene therapies have been carried out in experimental animals with colon cancer and liver cancer, and the results are encouraging.
Gene replacement therapy:
A gene named p53 codes for a protein with a molecular weight of 53 kilo Daltons (hence p53). p53 is considered to be a tumor-suppressor gene, since the protein it encodes binds with DNA and inhibits replication. The tumor cells of several tissues (breast, brain, lung, skin, bladder, colon, bone) were found to have altered genes of p53 (mutated p53), synthesizing different proteins from the original.
These altered proteins cannot inhibit DNA replication. It is believed that the damaged p53 gene may be a causative factor in tumor development. Some workers have tried to replace the damaged p53 gene by a normal gene by employing adenovirus vector systems .There are some encouraging results in the patients with liver cancer.
Gene Therapy for Aids:
AIDS is a global disease with an alarming increase in the incidence every year. It is invariably fatal, since there is no cure. Attempts are being made to relieve the effects of AIDS by gene therapy. Some of the approaches are discussed hereunder.
rev and env genes:
A mutant strain of human immunodeficiency virus (HIV), lacking rev and env genes has been developed. The regulatory and envelope proteins of HIV are respectively produced by rev and env genes. Due to lack of these genes, the virus cannot replicate.
Researchers have used HIV lacking rev and env genes for therapeutic purposes. T-Lymphocytes from HIV-infected patients are removed, and mutant viruses are inserted into them. The modified T-lymphocytes are cultivated and injected into the patients. Due to lack of essential genes, the viruses (HIV) cannot multiply, but they can stimulate the production of CD8 (cluster determinant antigen 8) cells of T-lymphocytes. CD8 cells are the killer lymphocytes. It is proved in the laboratory studies that these lymphocytes destroy the HIV-infected cells.
Genes of HIV proteins:
Some genes synthesizing HIV proteins are attached to DNA of mouse viruses. These genetically-modified viruses are injected to AIDS patients with clinical manifestations of the disease. It is believed that the HIV genes stimulate normal body cells to produce HIV proteins. The latter in turn stimulate the production of anti-HIV antibodies which prevent the HIV replication in AIDS patients.
Gene to inactivate gp120:
gp120 is a glycoprotein (molecular weight 120 kilo Daltons) present in the envelope of HIV. It is absolutely essential for binding of virus to the host cell and to bring replication. Researchers have synthesized a gene (called F105) to produce an antibody that can inactivate gp120.
In the anti- AIDS therapy, HIV-infected cells are engineered to produce anti-HIV antibodies when injected into the organism. Studies conducted in experimental animals showed a drastic reduction in the synthesis of gp120 due to anti-AIDS therapy. The production of F1IV particles was also very reduced. There are some attempts to prevent AIDS by antisense therapy.