ADVERTISEMENTS:
In this article we will discuss about:- 1. Meaning of Mutations 2. Characteristics of Mutations 3. Classification 4. Types 5. Agents 6. Detections 7. Nutritional Deficiency Method 8. Spontaneous Mutations 9. Applications of Mutations in Crop Improvement.
Contents:
- Meaning of Mutations
- Characteristics of Mutations
- Classification of Mutations
- Types of Mutations
- Agents of Mutations
- Detections of Mutations
- Nutritional Deficiency Method of Mutations
- Spontaneous Mutations
- Applications of Mutations in Crop Improvement
1. Meaning of Mutations:
ADVERTISEMENTS:
Mutation refers to sudden heritable change in the phenotype of an individual. In the molecular term, mutation is defined as the permanent and relatively rare change in the number or sequence of nucleotides. Mutation was first discovered by Wright in 1791 in male lamb which had short legs.
Later on mutation was reported by Hugo de Vries in 1900 in Oenothera, Morgan (1910) in Drosophila (white eye mutant) and several others in various organisms. The term mutation was coined by de Vries.
2. Characteristics of Mutations:
Mutations have several characteristic features.
ADVERTISEMENTS:
Some of the important characteristics of mutations are briefly presented below:
i. Nature of Change:
Mutations are more or less permanent and heritable changes in the phenotype of an individual. Such changes occur due to alteration in number, kind or sequence of nucleotides of genetic material, i.e., DNA in most of the cases.
ii. Frequency:
Spontaneous mutations occur at a very low frequency. However, the mutation rate can be enhanced many fold by the use of physical and chemical mutagens.
The frequency of mutation for a gene is calculated as follows:
Frequency of gene mutation = M / M + N
where, M = number of individuals expressing mutation for a gene, and
N = number of normal individuals in a population.
ADVERTISEMENTS:
iii. Mutation Rate:
Mutation rate varies from gene to gene. Some genes exhibit high mutation rate than others. Such genes are known as mutable genes, e.g., white eye in Drosophila. In some genomes, some genes enhance the natural mutation rate of other genes. Such genes are termed as mutator genes.
The example of mutator gene is dotted gene in maize. In some cases, some genes decrease the frequency of spontaneous mutations of other genes in the same genome, which are referred to as anti-mutator genes. Such gene has been reported in bacteria and bacteriophages.
iv. Direction of Change:
ADVERTISEMENTS:
Mutations usually occur from dominant to recessive allele or wild type to mutant allele. However, reverse mutations are also known, e.g., notch wing and bar eye in Drosophila.
v. Effects:
Mutations are generally harmful to the organism. In other words, most of the mutations have deleterious effects. Only about 0.1% of the induced mutations are useful in crop improvement. In majority of cases, mutant alleles have pleiotropic effects. Mutations give rise to multiple alleles of a gene.
vi. Site of Mutation:
ADVERTISEMENTS:
Muton which is a sub-division of gene is the site of mutation. An average gene contains 500 to 1000 mutational sites. Within a gene some sites are highly mutable than others. These are generally referred to as hot spots. Mutations may occur in any tissue of an organism, i.e., somatic or gametic.
vii. Type of Event:
Mutations are random events. They may occur in any gene (nuclear or cytoplasmic), in any cell (somatic or reproductive) and at any stage of development of an individual.
viii. Recurrence:
ADVERTISEMENTS:
The same type of mutation may occur repeatedly or again and again in different individuals of the same population. Thus, mutations are of recurrent nature.
3. Classification of Mutations:
Mutations can be classified in various ways. A brief classification of mutations on the basis of:
(1) Source,
(2) Direction,
(3) Tissue,
ADVERTISEMENTS:
(4) Effects,
(5) Site,
(6) Character, and
(7) Visibility is presented in Table 14.1.
4. Types of Mutants:
ADVERTISEMENTS:
The product of a mutation is known as mutant. It may be a genotype or an individual or a cell or a polypeptide.
There are four main classes of identifiable mutants, viz:
(i) Morphological,
(ii) Lethal,
(iii) Conditional and
(iv) Biochemical.
These are briefly described below:
i. Morphological:
Morphological mutants refer to change in form, i.e., shape, size and colour. Albino spores in Neurospora, curly wings in Drosophila, dwarf peas, short legged sheep are some examples of morphological mutants.
ii. Lethal:
In this class, the new allele is recognized by its mortal or lethal effect on the organism. When the mutant allele is lethal all individuals carrying such allele will die; but when it is semi-lethal or sub-vital some of the individuals will survive.
iii. Conditional Lethal:
Some alleles produce a mutant phenotype under specific environmental conditions. Such mutants are called restrictive mutants. Under other conditions they produce normal phenotype and are called permissive. Such mutants can be grown under permissive conditions and then be shifted to restrictive conditions for evaluation.
iv. Biochemical Mutant:
Some mutants are identified by the loss of a biochemical function of the cell. The cell can assume normal function, if the medium is supplemented with appropriate nutrients. For example, adenine auxotroph’s can be grown only if adenine is supplied, whereas wild type does not require adenine supplement.
5. Agents of Mutations:
Mutagens:
Mutagens refer to physical or chemical agents which greatly enhance the frequency of mutations. Various radiations and chemicals are used as mutagens. Radiations come under physical mutagens. A brief description of various physical and chemical mutagens is presented below:
Physical Mutagens:
Physical mutagens include various types of radiations, viz. X-rays, gamma rays, alpha particles, beta particles, fast and thermal (slow) neutrons and ultra violet rays (Table 14.2).
A brief description of these mutagens is presented below:
i. X-Rays:
X-rays were first discovered by Roentgen in 1895. The wavelengths of X-rays vary from 10-11 to 10-7. They are sparsely ionizing and highly penetrating. They are generated in X-rays machines. X-rays can break chromosomes and produce all types of mutations in nucleotides, viz., addition, deletion, inversion, transposition, transitions and trans-versions.
These changes are brought out by adding oxygen to deoxyribose, removing amino or hydroxyl group and forming peroxides. X-rays were first used by Muller in 1927 for induction of mutations in Drosophila.
In plants, Stadler in 1928 first used X-rays for induction of mutations in barley. Now X-rays are commonly used for induction of mutations in various crop plants. X-rays induce mutations by forming free radicals and ions.
ii. Gamma Rays:
Gamma rays are identical to X-rays in most of the physical properties and biological effects. But gamma rays have shorter wave length than X-rays and are more penetrating than X-rays. They are generated from radioactive decay of some elements like 14C, 60C, radium etc.
Of these, cobalt 60 is commonly used for the production of Gamma rays. Gamma rays cause chromosomal and gene mutations like X-rays by ejecting electrons from the atoms of tissues through which they pass. Now a days, gamma rays are also widely used for induction of mutations in various crop plants.
iii. Alpha Particles:
Alpha rays are composed of alpha particles. They are made of two protons and two neutrons and thus have double positive charge. They are densely ionizing, but lesser penetrating than beta rays and neutrons. Alpha particles are emitted by the isotopes of heavier elements.
They have positive charge and hence they are slowed down by negative charge of tissues resulting in low penetrating power. Alpha particles lead to both ionization and excitation resulting in chromosomal mutations.
iv. Beta Particles:
Beta rays are composed of beta particles. They are sparsely ionizing but more penetrating than alpha rays. Beta particles are generated from radioactive decay of heavier elements such as 3H, 32P, 35S etc. They are negatively charged, therefore, their action is reduced by positive charge of tissues. Beta particles also act by way of ionization and excitation like alpha particles and result in both chromosomal and gene mutations.
v. Fast and Thermal Neutrons:
These are densely ionizing and highly penetrating particles. Since they are electrically neutral particles, their action is not slowed down by charged (negative or positive) particles of tissues. They are generated from radioactive decay of heavier elements in atomic reactors or cyclotrons. Because of high velocity, these particles are called as fast neutrons.
Their velocity can be reduced by the use of graphite or heavy water to produce slow neutrons or thermal neutrons. Fast and thermal neutrons result in both chromosomal breakage and gene mutation. Since they are heavy particles, they move in straight line. Fast and thermal neutrons are effectively used for induction of mutations especially in asexually reproducing crop species.
vi. Ultraviolet Rays:
UV rays are non-ionizing radiations, which are produced from mercury vapour lamps or tubes. They are also present in solar radiation. UV rays can penetrate one or two cell layers. Because of low penetrating capacity, they are commonly used for radiation of micro-organisms like bacteria and viruses.
In higher organisms, their use is generally limited to irradiation of pollen in plants and eggs in Drosophila UV rays can also break chromosomes. They have two main chemical effects on pyrimidine’s.
The first effect is the addition of a water molecule which weakens the H bonding with its purine complement and permits localized separation of DNA strands. The second effect is to join pyrimidines to make a pyrimidine dimer.
This dimerization can produce TT, CC, UU and mixed pyrimidine dimers like CT. Dimerization interferes with DNA and RNA synthesis. Inter-strand dimers cross link nucleic acid chains, inhibiting strand separation and distribution.
Chemical Mutagens:
There is a long list of chemicals which are used as mutagens. Detailed treatment of such chemicals is beyond the scope of this discussion.
The chemical mutagens can be divided into four groups, viz:
(a) Alkylating agents,
(b) Base analogues,
(c) Acridine dyes, and
(d) Others (Table 14.3).
A brief description of some commonly used chemicals of these groups is presented below.
a. Alkylating Agents:
This is the most powerful group of mutagens. They induce mutations especially transitions and transversions by adding an alkyl group (either ethyl or methyl) at various positions in DNA. Alkylation produces mutation by changing hydrogen bonding in various ways.
The alkylating agents include ethyl methane sulphonate (EMS), methyl methane sulphonate (MMS), ethylene imines (EI), sulphur mustard, nitrogen mustard, etc.
Out of these, the first three are in common use. Since the effect of alkylating agents resembles those of ionizing radiations, they are also known as radiomimetic chemicals. Alkylating agents can cause various large and small deformations of base structure resulting in base pair transitions and transversions.
Transversions can occur either because a purine has been so reduced in size that it can accept another purine for its complement, or because a pyrimidine has been so increased in size that it can accept another pyrimidine for its complement. In both cases, diameter of the mutant base pair is close to that of a normal base pair.
b. Base Analogues:
Base analogues refer to chemical compounds which are very similar to DNA bases. Such chemicals sometimes are incorporated in DNA in place of normal base during replication. Thus, they can cause mutation by wrong base pairing. An incorrect base pairing results in transitions or transversions after DNA replication. The most commonly used base analogues are 5 bromo uracil (5BU) and 2 amino purine (2AP).
5 bromo uracil is similar to thymine, but it has bromine at the C5 position, whereas thymine has CH3 group at C5 position. The presence of bromine in 5BU enhances its tautomeric shift from keto form to the enol form. The keto form is a usual and more stable form, while enol form is a rare and less stable or short lived form. Tautomeric change takes place in all the four DNA bases, but at a very low frequency.
The change or shift of hydrogen atoms from one position to another either in a purine or in a pyrimidine base is known as tautomeric shift and such process is known as tautomerization.
The base which is produced as a result of tautomerization is known as tautomeric form or tautomer. As a result of tautomerization, the amino group (-NH2) of cytosine and adenine is converted into imino group (-NH). Similarly keto group (C = 0) of thymine and guanine is changed to enol group (-OH).
5BU is similar to thymine, therefore, it pairs with adenine (in place of thymine). A tautomer of 5BU will pair with guanine rather than with adenine. Since the tautomeric form is short-lived, it will change to keto form at the time of DNA replication which will pair with adenine in place of guanine.
In this way it results in AT GC and GC —> AT transitions. The mutagen 2AP acts in a similar way and causes AT <-> GC transitions. This is an analogue of adenine.
c. Acridine Dyes:
Acridine dyes are very effective mutagens. Acridine dyes include, pro-flavin, acridine orange, acridine yellow, acriflavin and ethidium bromide. Out of these, pro-flavin and acriflavin are in common use for induction of mutation. Acridine dyes get inserted between two base pairs of DNA and lead to addition or deletion of single or few base pairs when DNA replicates (Fig. 14.1).
Thus, they cause frameshift mutations and for this reason acridine dyes are also known as frameshift mutagens. Proflavin is generally used for induction of mutation in bacteriophages and acriflavin in bacteria and higher organisms.
d. Other Mutagens:
Other important chemical mutagens are nitrous acid and hydroxy amine. Their role in induction of mutation is briefly described here. Nitrous acid is a powerful mutagen which reacts with C6 amino groups of cytosine and adenine. It replaces the amino group with oxygen (+ to – H bond). As a result, cytosine acts like thymine and adenine like guanine.
Thus, transversions from GC —> AT and AT —> GC are induced. Hydroxylamine is a very useful mutagen because it appears to be very specific and produces only one kind of change, namely, the GC —> AT transition. All the chemical mutagens except base analogues are known as DNA modifiers.
6. Detection of Mutation:
Detection of mutations depends on their types. Morphological mutations are detected either by change in the phenotype of an individual or by change in the segregation ratio in a cross between normal (with marker) and irradiated individuals. The molecular mutations are detected by a change in the nucleotide, and a biochemical mutation can be detected by alteration in a biochemical reaction.
The methods of detection of morphological mutants have been developed mainly with Drosophila. Four methods, viz., (1) CIB method, (2) Muller’s 5 method, (3) attached X-chromosome method, and (4) curly lobe plum method are in common use for detection of mutations in Drosophila.
A brief description of each method is presented below:
i. CIB Method:
This method was developed by Muller for detection of induced sex linked recessive lethal mutations in Drosophila male. In this technique, C represents a paracentric inversion in large part of X-chromosome which suppresses crossing over in the inverted portion. The I is a recessive lethal. Females with lethal gene can survive only in heterozygous condition.
The B stands for bar eye which acts as a marker and helps in identification of flies. The I and B are inherited together because C does not allow crossing over to occur between them. The males with CIB chromosome do not survive because of lethal effect.
The important steps of this method are as follows:
(a) A cross is made between CIB female and mutagen treated male. In F1 half of the males having normal X-chromosome will survive and those carrying CIB chromosome will die. Among the females, half have CIB chromosome and half normal chromosome (Fig. 14.2). From F1, females with CIB chromosome and male with normal chromosome are selected for further crossing.
(b) Now a cross is made between CIB female and normal male. This time the CIB female has one CIB chromosome and one mutagen treated chromosome received from the male in earlier cross.
This will produce two types of females, viz., half with CIB chromosome and half with mutagen treated chromosome (with normal phenotype). Both the progeny will survive. In case of males, half with CIB will die and other half have mutagen treated chromosome.
If a lethal mutation was induced in mutagen treated X-chromosome, the remaining half males will also die, resulting in absence of male progeny in the above cross. Absence of male progeny in F2 confirms the induction of sex linked recessive lethal mutation in the mutagen treated Drosophila male.
ii. Muller 5 Method:
This method was also developed by Muller to detect sex linked mutation in Drosophila. This method is an improved version of CIB method. This method differs from CIB method in two important aspects. First, this method utilizes apricot recessive gene in place of recessive lethal in CIB method. Second, the female is homozygous for bar apricot genes, whereas it is heterozygous for IB genes in CIB method.
In this method, the mutation is detected by the absence of wild males in F2 progeny. This method consists of following important steps (Fig. 14.3).
a. A homozygous bar apricot female is crossed with mutagen treated male. In F1 we get two types of progeny, viz., heterozygous bar females and bar apricot (Muller) males.
b. These F1 are inter-mated. This produces four types of individuals. Half of the females are homozygous bar apricot, and half are bar heterozygous. Among the males, half are bar apricot (Muller 5) and half should be normal. If a lethal mutation is induced, the normal male will be absent in the progeny.
iii. Attached X-Method:
This method is used to detect sex linked visible mutations in Drosophila. In this method a female in which two X-chromosomes are united or attached together is used to study the mutation (Fig. 14.4). Therefore, this method is known as attached X-method. The attached X females (XXY) are crossed to mutagen treated male. This cross gives rise to super females (XX-X), attached female (XXY), mutant male (XY) and YY.
The YY individuals die and super female also usually dies. The surviving male has received X-chromosome from mutagen treated male and Y chromosome from attached X-female. Since Y chromosome does not have corresponding allele of X-chromosome, even recessive mutation will express in such male which can be easily detected.
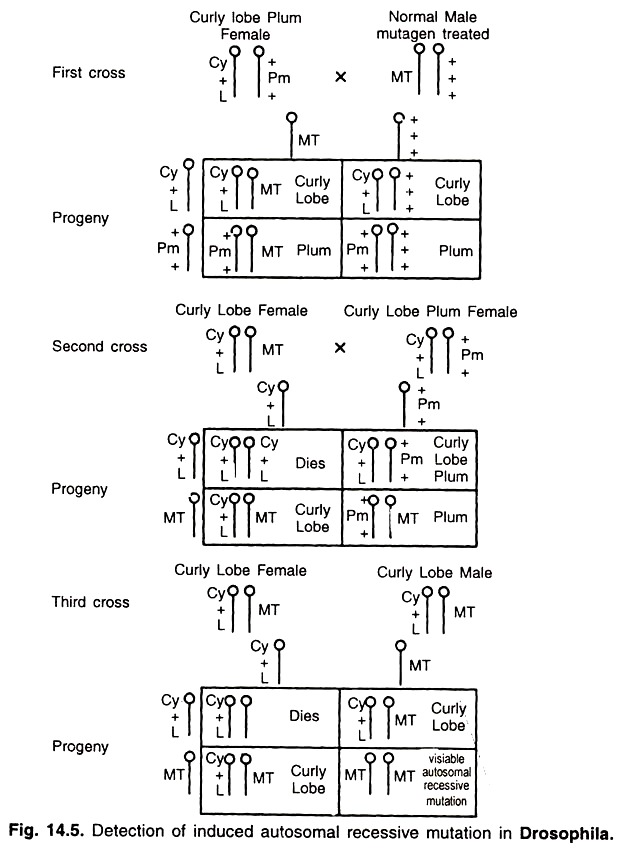
iv. Curly Lobe-Plum Method:
This method is used for detection of mutation in autosomes. In this method curly refers to curly wings, lobe to lobed eye and plum to plum or brownish eye. All these three genes are recessive lethal. Curly (CY) and lobed (L) genes are located in one chromosome and plum (Pm) in another but homologous chromosome.
Crossing over between these chromosomes cannot occur due to presence of inversion. Moreover, homozygous individuals for CYL or Pm cannot survive because of lethal effect. Only heterozygotes survive. Thus, this system is also known as balanced lethal system. This method consists of following steps (Fig. 14.5).
a. A cross is made between curly lobe plum (CYL/Pm) female and mutagen treated male. This produces 50% progeny as curly lobe and 50% as plum.
b. In the second generation cross is made between curly lobe female and curly lobe plum male. This will give rise to curly lobe plum, curly lobe and plum individuals in 1 : 1 : 1 ratio and homozygous curly will die due to lethal effect. From this progeny, curly lobe females and males are selected for further mating.
c. In third generation, a cross is made between curly lobe female carrying one mutagen treated autosome and curly lobe male also carrying treated autosome. This results in production of 50% progeny as curly lobe, 25% homozygous curly lobe which die and 25% progeny homozygous for treated autosomes.
This will express as autosomal recessive mutation and constitute one third of the surviving progeny. A comparison of different methods of detection of mutation in Drosophila is given in Table 14.4.
Detection of Mutations in Plants:
As stated earlier, the techniques of detection of induced mutations have been mostly developed on Drosophila. In plants, such techniques have not been developed properly. In plants, two methods are used for detection of mutations depending upon the visibility of mutations.
These methods are briefly described below:
i. Detection of Visible Mutations:
Visible mutations generally occur in qualitative or oligogenic characters. Such mutations are detected on the basis of altered phenotype.
This technique consists of following steps:
a. The seeds are treated with a mutagen. For this purpose an improved variety or strain is used.
b. The treated seeds are grown in the experimental field. These plants are known as M1 plants or M1 generation. These M1 plants are selfed to avoid outcrossing. The seeds obtained from M1 plants represent M2 generation of seed.
c. The seeds obtained from M1 plants are grown to obtain M2 plants. A sufficiently large population should be raised in M2 generation to obtain mutant phenotypes which generally occur at a low frequency.
d. A search is made to identify or to detect plants which differ from the parent variety. Such plants are isolated and their frequency is estimated. Such mutations are called macro- mutations.
In maize, a different procedure is used for detection of visible mutations. In maize, some stocks are homozygous for several recessive genes and other stocks are homozygous for several dominant genes. The seeds of homozygous dominant lines are treated with a mutagen and M1 plants are raised. These M1 plants are crossed with homozygous recessive stock.
The mutagen treated plants are used as females due to presence of some degree of male sterility in these plants as a consequence of mutagenic effect. The F1 progeny of such cross is grown and a search is made to detect plants with recessive phenotype for a specific gene. Presence of plants with recessive phenotype for a gene confirms induction of mutation.
ii. Detection of Invisible Mutation:
Invisible mutations usually occur in quantitative or polygenic characters like yield and protein content. Detection of such mutations requires quantitative measurement of such characters. For yields, the mutagen treated and untreated variety is grown in replicated trials.
If the yield of treated and untreated treatments differs significantly, the presence of mutation is indicated. Similarly, if the protein content of treated material differs significantly from the parent variety, it indicates that mutation has taken place. Such mutations are called as micro-mutations.
7. Nutritional Deficiency Method of Mutations:
This method of detection of induced mutations is used in micro-organisms like Neurospora. The normal strain is treated with a mutagen and then cultured on minimal medium. A minimal medium contains sugar, salt, inorganic acids, nitrogen and vitamin biotin. The normal strain of Neurospora grows well on the minimal medium, but a biochemical mutant fails to grow on such medium.
This confirms induction of mutation. Then minimal medium is supplemented with certain vitamins or amino acids, one by one and the growth is observed. The medium which results in normal growth of mutagen treated mould indicates that the mutant lacks synthesis of that particular vitamin or amino acid, addition of which to the minimal culture medium has resulted in normal growth of treated strain.
8. Spontaneous Mutations:
Naturally occurring mutations are known as spontaneous mutations. Such mutations are induced by chemical mutagens or radiations which are present in the external environment to which an organism is exposed. Temperature also affects the frequency of spontaneous mutations. A rise of 10°C in the temperature leads to fivefold increase in mutation rate in an organism exposed to such variation in temperature.
Drastic change of temperature in any direction produces still greater effect on mutation frequency. External environmental conditions of any type, i.e., either extremely high or low leads to increase in the mutation frequency.
Internal environment of an organism also plays an important role in the induction of spontaneous mutations. For example, spontaneous rearrangements of DNA bases result in base pair transitions. Similarly, errors in DNA repair or replication can cause spontaneous mutations.
9. Applications of Mutations in Crop Improvement:
Induced mutations are useful in crop improvement in five principal ways, viz:
(1) Development of improved varieties,
(2) Induction of male sterility,
(3) Production of haploids,
(4) Creation of genetic variability, and
(5) Overcoming self-incompatibility.
These are briefly discussed below:
ADVERTISEMENTS:
i. Development of Improved Varieties:
More than 2000 improved varieties (some directly and some by use of mutants in hybridization) have been developed through induced mutations in various field crops all over the world.
In India, induced mutations have been instrumental in developing improved varieties in wheat (NP 836, Sarbati Sonor’a, Pusa Lerma), barley (RDB 1), rice (Jagannath, IIT 48, NT 60), tomato, castor bean (Aruna, Sobhagya), cotton (MCU 7, MCU 10, Indore 2), groundnut (TGI), sugarcane (Co 8152, 8153) and several other crops.
Besides high yield, varieties have been developed with better quality, earliness, dwarfness, disease resistance and low toxin contents in various crops.
Improvement in quality has been achieved for protein content in wheat and rice, oil content in mustard and sugar content in sugarcane. Earliness has been achieved in castor (from 270 days to 140 days), rice and soybean. Dwarf varieties have been developed through the use of mutant parents in wheat, rice, Sorghum and pearl millet.
Disease resistance has been induced in oats to Victoria blight and crown rust; in wheat for strip rust; in barley for mildew; in groundnut for leaf spot and stem rust; in sugarcane for red rot; in apple for mildew, etc. Low toxin content varieties have been developed in rapeseed and mustard for erusic acid and in Lathyrus sativa for neurotoxin content.
ii. Induction of Male Sterility:
Induced mutations have been useful in induction of male sterility in some crop plants. Genetic male sterility has been induced in durum wheat using radiations. CMS mutants have been induced in barley, sugarbeet, pearl millet and cotton. Use of GMS and CMS lines helps in reducing the cost of hybrid seed production.
iii. Production of Haploids:
Use of X-ray irradiated pollens has helped in production of haploids in many crops. Chromosome doubling of these haploids results in the development of inbred lines which can be utilized in the development of commercial hybrids.
iv. Creation of Genetic Variability:
Induced mutations are very effective in creating genetic variability for various economic characters in crop plants. Induced mutations have been used for increasing the range of genetic variability in barley, oats, wheat and many other crops. In asexually propagated crops like sugarcane and potato, somatic mutations may be useful, because the mutant plant can be multiplied as a clone.
v. Overcoming Self-Incompatibility:
Mutation of S gene by irradiation offers a solution to the production of self-fertile plants in self-incompatible species. This has been successful in case of Prunusovium. Besides this practical application in crop improvement, induced mutations are of fundamental interest in genetical studies.
Induced mutations have some limitations also. Most of the mutations are deleterious and undesirable. Identification of micro-mutations, which are more useful to a plant breeder is usually very difficult. Since mutations are produced at a very low frequency, a very large plant population has to be screened to identify and isolate desirable mutants.