ADVERTISEMENTS:
The following points highlight the four methods to detect and isolate mutants. The methods are: 1. Replica Plating Technique 2. Resistance Selection Method 3. Substrate Utilization Method 4. Carcinogenicity Test.
1. Replica Plating Technique:
Lederberg and Lederberg (1952) have given replica plating technique. This technique is used to detect auxotrophic mutants which differentiates between mutants and wild type strains on the basis of ability to grow in the absence of an amino acid.
However, isolation of a leucine auxotroph through replica plating follows the following steps (Fig. 9.15):
(i) Generate the mutants by treating a culture with a mutagen e.g. nitrosoguanidine.
(ii) Inoculate a plate containing complete growth medium and incubate it at proper temperature. Both wild type and mutant survivors will form colonies on the complete medium. This plate containing complete medium is called master plate.
(iii) Prepare a piece of sterile velvet and gently press on upper surface of master plate to pick up bacterial cells from each colony. As pressed on master plate, again gently press the velvet on replica plates containing complete medium in one set and lacking only leucine in the other set. Thus, the bacterial cells are transferred in replica plates in the same position as in master plate.
(iv) Incubate the plates and compare the replica plates with master plate for the bacterial colony not growing on replica plate. The leucine auxotrophs (Leu–) will not grow on replica plates devoid of leucine. Isolate and culture Leu– cells growing on complete medium.
ADVERTISEMENTS:
Replica plating can also be used to isolate the temperature sensitive mutants. It involves by forming colonies at 30°C and then transferring these colonies on two plates, one incubated at 30°C and the other at 42°C. The colony which grow at 30°C and absent at 42°C certainly consist of temperature sensitive mutation.
2. Resistance Selection Method:
It is the other approach for isolation of mutants. Generally the wild type cells are not resistant either to antibiotics or bacteriophages. Therefore, it is possible to grow the bacterium in the presence of the agent (antibiotics or bacteriophage and look for survivors. This method is applied for isolation of mutants resistant to any chemical compounds that can be amended in agar, phage resistant mutants.
3. Substrate Utilization Method:
This method is employed in the selection of bacteria. Several bacteria utilize only a few primary carbon sources. The cultures are plated onto medium containing an alternate carbon sources. Any colony that grows on medium can use the substrate and are possibly mutants. These can be isolated.
Sugar utilization mutants are also isolated by means of colour indicator plates. A popular medium (EMB agar) is used for this purpose. The EMB agar contains two dyes eosin and methylene blue in the medium. Colour of these dyes is sensitive to pH. This medium also contains lactose sugar as carbon source and complete mixture of amino acids.
Therefore, both lactose wild type (Lac+) and lactose mutant (Lac–) cells can grow and form colonies on EMB agar plates. The Lac+ cells catabolize lactose and secrete acids, therefore, local pH of the medium decreases. This results in staining of colony to dark purple.
On the other hand, Lac– cells are unable to utilize lactose and use some of the amino acids as carbon source. After utilization of amino acid, possibly ammonia is produced that increases the local pH and decolorizes the dye resulting in white colony.
4. Carcinogenicity Test:
An understanding has developed to identify the environmental carcionogens that cause mutation and induce cancer in organisms. This method is based on detecting potential of carcinogens and testing for mutagenicity in bacteria.
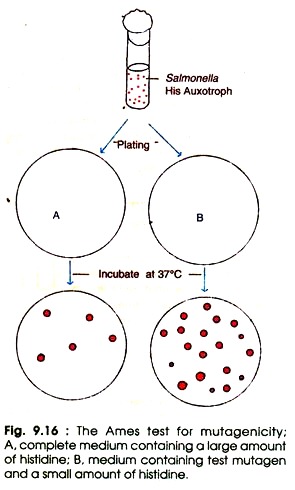
Ames (1973) developed a method for deletion of mutagenicity of carcinogens which is commonly known as Ames test. It is widely used to detect the carcinogens. The Ames test is a mutational reversion assay in which several special strains of Salmonella typhimurium are employed. Each strain contains a different mutation in the operon of histidine biosynthesis.
The Ames test follows the following steps (Fig. 9.16):
ADVERTISEMENTS:
(i) Prepare the culture of Salmonella histidine auxotrophs (His–).
(ii) Mix the bacterial cells and test substance (mutagen) in dilute molten top agar with a small amount of histidine in one set, and control with complete medium plus large amount of histidine.
(iii) Pour the molten mix on the top of minimal agar plates and incubate at 37°C for 2-3 days. Until histidine is depleted all the His– cells will grow in the presence of test mutagens. When histidine is completely exhausted only the reverants (the mutants which have regained the original wild type characters) will grow on agar plate.
ADVERTISEMENTS:
The number of spontaneous reverants is low, whereas the number of reverants induced by the test mutagen is quite high. In order to estimate the relative mutagenicity of the mutagenic substance the visible colonies are counted and compared with control. The high number of colonies represents the greater mutagenicity.
A mammalian liver extract is added to the above molten top agar before plating. The extract converts the carcinogens into electrophilic derivatives which will soon react with DNA molecule.
In natural way this process occurs in mammalian system when foreign substances are metabolized in the liver. Bacteria do not possess the metabolizing capacity as liver does; therefore, the liver extract is added to this test, just to promote the transformation.
Therefore, several potential carcinogens that generally are not carcinogenic until modified in liver, for example aflatoxins viz., B1, B2, G1, G2, etc. The Ames test has now been used with thousands of substances and mixtures such as the industrial chemicals, food additives, pesticides, hair dyes and cosmetics.