ADVERTISEMENTS:
This article throws light upon the top four methods of gene transfer.
The top four methods of gene transfer are: (1) DNA Transfer in Protoplasts (2) Free DNA Transfer to Intact Tissue (3) Agrobacterium Mediated Gene Transfer Method and (4) Integration and Expression.
The first transgenic plant was produced via Agrobacterium mediated modified transformation of Nicotiana tabacum protoplasts by Horsch and co-workers in 1984. Since then several dozen plant species have been genetically engineered using different techniques.
ADVERTISEMENTS:
Simultaneous development of other techniques such as selectable markers facilitated the development in genetic engineering for obtaining transformed plants. But this technique is not suitable for monocotyledon plants as they are not natural host of Agrobacterium (There is evidence that limited gene transfer is possible in monocots by this system).
Therefore, other methods of direct gene transfer have been developed for use with monocots and other species. These can be categorized on the basis of the use of protoplasts or cell and tissue as the target materials. Freshly isolated protoplasts are used for genetic transformation. Protoplast stage is for a short duration, before it regenerates cell wall, and DNA transfer is performed during this period.
I. DNA Transfer in Protoplasts:
(i) Electroporation
ADVERTISEMENTS:
(ii) Chemically stimulated DNA uptake by protoplasts
(iii) Liposomes
(iv) Micro-injection
(v) Sonication
II. DNA Transfer in Plant Tissues:
(i) Acceleration of DNA coated micro-particles
(ii) Laser micro-beam
(iii) Silicon carbide fibres
Direct uptake of DNA by isolated protoplasts is a genotype dependent response. Regeneration from protoplasts is not common in all the species. Due to this, production of fertile transgenic plants has remained difficult in most of the cereal species. However, transgenic fertile plants have been produced in Sorghum vulgare, Oryza sativa and Hordeum vulgare. Direct delivery of free DNA molecules into plant protoplasts by physical (electroporation and micro injection) and chemical (polyethylene glycol) methods have been developed to facilitate DNA delivery across the plasma membrane.
Method # 1. DNA Transfer in Protoplasts:
Electroporation:
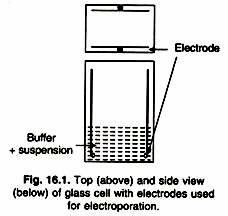
This method is based on the use of the short electrical pulses of high field strength. Electroporation causes the uptake of DNA into protoplasts by temporary permeabilization of the plasma membrane to macromolecules. Protoplasts and foreign DNA are placed in a buffer between two electrodes and a high intensity electric current is passed, the alternating current of about 1 MHz is applied to align the protoplast by di-electrophoresis.
ADVERTISEMENTS:
Once aligned, fusion is induced by applying one or more direct current pulses (1-3kV /cm, 10-100 (is), then the alternating field is reapplied briefly to maintain close membrane contact for fusion (Fig. 16.1). Electric field damages membranes and creates pores in membranes. DNA diffuses through these pores immediately after the electric field is applied, until the pores are resealed. Technique is optimized by using appropriate electric field strength (defined as the applied voltage divides by the distance between two electrodes).
The optimum field strength is dependent on the followings:
1. The pulse length of electric current
ADVERTISEMENTS:
2. Composition and temperature of the buffer solution
3. Concentration of foreign DNA in the suspension
4. Protoplasts density, and
5. Size of the protoplasts.
ADVERTISEMENTS:
It has been demonstrated that the removal of pectin from the plant wall increases the amount of DNA which can be introduced by electroporation. Tobacco mosaic virus was introduced in tobacco protoplasts by this method. Electroporation has been used successfully for transient (when foreign gene which is present in cell but not integrated in the chromosome, shows expression in cytoplasm) and stable transformation (foreign gene integration in host chromosome and is expressed) of protoplasts from a wide range of species.
Plating efficiency (i.e., number of colonies recovered out of number of cells transferred on plates) of electroporated protoplasts grown on selection medium (containing selective marker) can be as high as 0.5%. The highest plant transformation efficiencies have been reported for tobacco, with 0.2% of electroporated leaf mesophyll protoplasts giving rise to transgenic calli. Low transformation efficiency is common in cereals, e.g., in rice 0.002% efficiency was recorded.
Chemically Stimulated DNA Uptake:
Direct uptake of DNA by protoplasts is stimulated by polyethylene glycol (PEG) and PEG is the most widely used chemical for this purpose. PEG mediated transformation involves mixing of freshly isolated protoplasts with DNA and immediately adding 15-20% PEG dissolved in a buffer containing divalent cations. This mixture is incubated for 30 minutes; protoplasts are washed and then plated in Petri plates for culture and growth.
ADVERTISEMENTS:
The optimization of transformation frequencies by this method include factors that follows:
1. PEG concentration in the mixture.
2. Composition and concentration of salts used.
3. The pH of the solution.
4. Concentration of the foreign DNA.
5. Size and form (linear, super-coiled) of the DNA molecules used.
ADVERTISEMENTS:
6. Culture and selection techniques used for protoplasts.
PEG mediated transformation is generally preferred over electroporation for stable transformation of monocot protoplasts due to relatively higher survival rates after treatment.
PEG also stimulates the uptake of liposomes and improves the efficiency of electroporation. PEG causes precipitation of ionic macromolecules like DNA and stimulates their uptake by
endocytosis. PEG mediated DNA uptake typically transforms 0.1 to 0.4% of the total protoplasts treated. Production of transgenic plants depends upon the regeneration competence of the transformed protoplasts.
In case of Petunia, 40% transformed calli derived from mesophyll protoplasts could be induced to form fertile plants. This is equal to about 0.1% transformation efficiency of the treated protoplasts. In different species different transformation efficiency has been observed, e.g., in embryogenic protoplasts suspension of rice was 0.0004% and, soya-bean and tobacco was 0.7-1%.
Liposomes:
Liposomes have also been used as a carrier for the introduction of nucleic acid into plant protoplasts. Liposomes are small lipid sacs containing plasmids and are prepared artificially. The fusion of liposomes with plant protoplasts is stimulated by chemicals such as PEG (endocytosis). Liposomes mediated transformation has been achieved by including positively charged agents such as cations in the transformation mixture or using the cationic liposome preparation (Fig. 16.2).
 Other chemical agents like polycation Polybrene or lipofectin have also been used for both transient and stable transformation for maize protoplast. Cationic liposome and polycation mediated DNA delivery are new protoplasts transformation methods and are considered better than other methods of transformation. There are several advantages for the use of this technique.
Other chemical agents like polycation Polybrene or lipofectin have also been used for both transient and stable transformation for maize protoplast. Cationic liposome and polycation mediated DNA delivery are new protoplasts transformation methods and are considered better than other methods of transformation. There are several advantages for the use of this technique.
1. Protection of DNA/RNA from nuclease digestion.
ADVERTISEMENTS:
2. They have low cell toxicity.
3. Encapsulation of nucleic acids makes them more stable during storage.
4. High degree of reproducibility.
5. This method is applicable to wide range of plant cell types.
Microinjection:
Delivery of nucleic acids to protoplasts or intact cells via microinjection is a labour intensive procedure that requires special capillary needles, pumps, micromanipulators, inverted microscope and other equipment. However, injection into the nucleus or cytoplasm is possible and cells can be cultured individually to produce callus or plants.
In this way selection of transformants by drug resistance or marker genes may be avoided. This method involves skill of the worker to insert needle into the cytoplasm or in the nucleus. The basic technique is similar to that used for animal cell microinjection. In order to microinject protoplasts or other plant cells, the cells need to be immobilized (Fig. 16.3).
The cells are immobilized by:
1. The use of a holding pipette which holds the cells by vacuum.
2. Attachment of cells to poly-L-lysin coated cover slips.
3. Embedding the cells in agarose, agar or sodium alginate.
Glass micropipette are prepared to have openings of about 0.3 µM in diameter and are inserted into plant cell cytoplasm and nuclei with the aid of a micromanipulators device. A syringe like device is used for the controlled delivery of volume (10-11 – 10-4 1) into the plant cell.
Most plant cells are injected while keeping inside micro-droplets (2-50 µl) of medium using a chamber which is sterile, vibration free and permits temperature and humidity regulation. A maximum of 100-200 cells per hour can be microinjected by this method.
The recovery of trans-formants is dependent upon the regeneration ability of the microinjected cells. Different methods have been used to grow injured (microinjected) single cells or protoplasts. Hanging droplets, covered under thin layer of agar or agarose, and micro-culture have been used (Fig. 16.4). Attempts have been made to inject linear or super-coiled DNA, in cytoplasm or in nucleus. Nuclear injections are found better for transformations.
Sonication:
Mild sonication (20 KHz ultrasound) has been used to facilitate the uptake and transient expression of a chloramphenicol acetyltransferase (CAT) gene in protoplasts of sugar beet (Beta vulgaris) and tobacco. This method was found superior than electroporation method used for the same material. Plating efficiency was also similar to untreated cells. However, transgenic plant production using this technique has not been reported so far.
Method # 2. Free DNA Transfer to Intact Tissue:
Acceleration of DNA Loaded Micro-particles (Particle Gun or Biolistic® Method):
This is latest technology to transfer DNA into intact tissues. Several devices are developed using different methods. All to achieve the transfer of micro-sized particles (micro-projectiles) coated with DNA to penetrate the cells. In this procedure micron size tungsten or gold particles are accelerated in a gun barrel to velocities sufficient for non-lethal penetration of cell walls and membranes.
Klein and co-workers in 1987 developed and used for the first time the particle gun to transfer chimeric DNA and viral RNA molecules into intact onion cells. Tungsten acted as a carrier of nucleic acids because it was available in micro-size balls, non-toxic to cells, and dense enough (high density) for rapid penetration of target material. Micro-projectile mediated transformation is a mechanical method of introducing DNA in to any plant species. This method can be successfully used where plasmids or protoplasts mediated transformation cannot be used.
An acceleration device is used to propel particles (micro projectiles) carrying plasmid DNA is called by various names based on machine or technique used to accelerate the particles such as ‘particle gun technology’, ‘biolistic method’, ‘DNA bombardment’, ‘particle acceleration of DNA method’ and ‘electric discharge particle acceleration method’.
This is a quick method of stable transformation and testing a gene for cell and organelle specific expression, this technique has three components:
1. The basic equipment to generate particle acceleration.
2. Metal particles coated with precipitated DNA (desired gene).
3. Plant tissues to be used for particle penetration. The method for regeneration should be previously standardized and proper tissues be selected for bombardment.
(a) Instrument:
The instrument is commercially available. Prototype was designed by Klein and co-workers. It uses the explosive force of gun powder (0.22 caliber gun cartridge) to accelerate a polypropylene cylindrical macro-projectile. Thin piece of polypropylene macro-projectile is loaded with micro-projectiles coated with DNA. Gun powder explosion forces thin macro-projectile to move with high speed toward another end of barrel, where it is blocked by a polycarbonate disc having an aperture.
Macro-projectile is stopped but micro-projectiles move fast through the aperture towards tissue placed in the same direction. For each transfer 50 µg tungsten is accelerated up to 2000 ft per second in a partial vacuum. With this speed, particles reach up to lower layers of cells in target tissues (Fig. 16.5).
The other devices are similar in basic design concept but use different methods to accelerate particles like use of compressed air or gas. Compressed air (130 kg/cm2 pressure) has been used to accelerate micro-projectiles at velocities (approximately 440 m/sec) necessary to achieve DNA delivery to plant cells.
An electric discharge particle acceleration device differs in basic design from the above described devices. In this device, a high voltage discharge (14 KV current) delivered to a small water droplet which quickly vaporizes and releases energy to propel DNA coated gold spheres into target cells (Fig. 16.6).
In similar way to above devices, a DNA carrier is attracted (accelerated) due to potential differences, stopped in-between by a screen, DNA coated particles cross the screen and fly towards target tissue and deliver the DNA into cells.
(b) DNA coating:
This is a sophisticated technology and requires precise preparation of DNA coated gold or tungsten particles. The particles should have following properties.
1. High density (19 g/cm2 or greater) to ensure proper acceleration and penetration through cell walls.
2. Size (0.5-5 µm) should match with size of the cells. Large sized spheres can be used with large cell size.
3. Gold is costlier than tungsten, but it does not oxidize like tungsten. DNA is precipitated onto the particles prior to bombardment. Most commonly, CaCl2 and spermidine are used to precipitate plasmid DNA (the desired gene attached to plasmid is suitable for integration into plant genome) onto tungsten particles. Ethanol is also used for precipitation of the DNA on gold particles.
(c) Plant tissue:
Plant tissue used for transformation should be competent to regenerate. Mostly embryogenic tissue is an ideal material for transformation (e.g., in corn, cotton, soya-bean, papaya and wheat) but transformed plants have been obtained after leaf bombardment in tobacco, and stem bombardment in cranberry. Normally, reporter gene and selectable marker genes are used to isolate and select transformed cells/plantlets.
Laser Micro-beam:
Weber and co-workers (1988) demonstrated use of laser beam for transformation of plant cells. An ultraviolet (UV) laser micro-beam has been used to introduce DNA into plant cells and chloroplasts. A 343 nm beam (wavelength of UV is 200 to 400 nm) is directed through an adjustable attenuator into the optical path of an inverted microscope.
The focus of the laser beam is adjusted so that it is identical with that of the objective lens. The laser beam is targeted by focusing on a specimen in the microscope. This laser beam can then make holes in any part of cell which is in focus. Laser micro-puncture of the cell wall and plasma membrane allows uptake (entry) of plasmid DNA into cells.
Brassica napus (rapeseed) cells and microspores have been used for transformation by this technique. This technique has also been used to transfer genes into isolated chloroplasts and chloroplasts of intact protoplasts. 20% transformation was achieved by this method but fertile plants are yet to be produced by this method.
Silicone Carbide Fibres:
Microinjection and electroporation methods have also been used for transfer of DNA using intact plant cells and tissues. Similarly, vortexing plasmid DNA and plant cells with silicon carbide fibre (0.6 µm in diameter and 10-80 µm in length) produced transformed cells at low frequency. Under vortex (vigorous shaking by vibration), silicone fibres penetrate cells and create fine holes permitting entry of DNA.
DNA uptake by imbibition’s in dried embryos of cereals and legume species has also been reported. This is a very simple method and dried somatic embryos can also be used for this method. Only transient gene expression has been observed and stable transformations are yet to be achieved by this method. Attempts have been made to introduce gene in pollen grains by microinjection in the anthers. These pollen grains can be used for fertilization to obtain transgenic plants.
Method # 3. Agrobacterium Mediated Gene Transfer Method:
Transgenic plants are produced by two methods:
(i) Indirect gene transfer using plasmid as a vector and
(ii) Direct gene transfer. Indirect gene transfer requires construction of a vector for carrying foreign genes based on Ti or Ri plasmids.
Vectors are the carrier DNAs into which ‘foreign’ DNAs or genes of interest are inserted to make a recombinant DNA (rDNA). Vectors along with this ‘foreign’ DNA (i.e., rDNA) are then introduced into appropriate host cell. Vectors are of two types-cloning vectors [used for obtaining millions of copies (cloning) of DNA segment] and expression vectors [used for expression of cloned gene to produce the product (protein)].
Plasmids are most commonly used vectors in gene cloning work. Isolation and purification of plasmids is a very routine experimental procedure. Several methods are available for isolation and purification of plasmid. The most critical stage in the process is lysis of the cell wall which is just sufficient for isolation of plasmid DNA without contamination by chromosomal DNA. Clear lysate contains plasmid. Alkali-SDS lysis and rapid boiling procedures are commonly used methods for plasmid isolation.
Most vectors carry marker genes which allow recognition e.g., antibiotic resistance – selectable markers, e.g., npt II (kanamycin resistance). Other features include (i) multiple unique restriction sites – a synthetic poly linker and (ii) bacterial origin of replication e.g. (Col El). The problem is that a vector having these properties may not be easy to transfer to plant system. Therefore, Agrobacterium Ti plasmid is preferred because of (1) wide host range and (2) presence of T-DNA border sequence. Plant cells do not have any endogenous plasmid. The plasmid vectors used for gene transfer in plants are based on pTi or pRi (tumour inducing or root inducing plasmid) present in Agro-bacterium tumefactions or A. rhizogenes.
Structure of T-DNA is described in the chapter -14. Presence of auxin and cytokinin producing genes caused tumour formation in plants, when T-DNA present in pTi is transferred in plant cell.
Therefore, these tumour forming genes are removed from T-DNA (called as disarming the plasmid) and gene of interest is introduced in that place (between left and right border of T-DNA). Thus this recombinant T-DNA is ready to transfer a gene by natural mechanism of gene transfer in a dicot host cell.
Since pTi or pRi are large plasmids, modified plasmids (co-integrative and binary) are prepared from Ti plasmid and used as described later on in this chapter. Thus by placing foreign genes into T-DNA region of Ti-plasmid, it is possible to clone (make copies) the introduced genes with the multiplication of plasmid residing inside the bacteria (self replication of plasmid makes millions of copies) which is grown on a medium and with the multiplication of bacterial population, residing plasmid is also multiplied by this method. It is possible to exploit the natural ability of Agrobacterium to transfer new DNA into the plant genome.
T-DNA: Transfer Mechanism:
Though exact mechanism of T-DNA transfer is not clearly known, effective role of vir region is known in the process of transfer. Genes in the vir region is activated by acetosyringone, a phenolic substance secreted by wounded cells of the host.
The phenolic signal molecules binds to the vir A gene product, the vir A proten (Fig. 16.7). As consequence of this, several vir rgion genes are activated and these gene products (proteins) help in copy of T-DNA and its insertion into host cell.
i. Nicking between 3rd and 4th base of 25 bp repeats (bottom strand). Vir-D operon encodes for an endonuclease that cause nick formation.
ii. Initiation of DNA synthesis in 5′-3′ direction.
iii. Involvement of bacterial genome – synthesis and secretion of glucose, cellulose, fibrils, and cell surface proteins. This is common physiological response in all soil bacteria and is involved with pathogenic characters.
The generation of the T-strand is the first step in the complex process of Agro-bacterium mediated plant cell transformation. Following its formation, this DNA must pass through the bacterial cell membrane, the bacterial cell wall, the plant cell wall and plant cell and nuclear membranes. Once inside the nucleus, the T-DNA must finally integrate stably into the plant cell genome (Fig. 16.8). During this entire transit process, the T-DNA strand also must avoid degradation by nuclease. The T-DNA exists as a DNA protein complex. The T-DNA complex protects it and mediates its travel.
The final step in the genetic transformation of plant cell is integration of T-DNA copy, presumably T-strand, into plant cell DNA. It has been argued that the T-strand might be converted to a double stranded (ds) DNA prior to integration.
Vectors Based on Ti and Ri Plasmids:
The Ti or Ri-plasmid cannot be used directly. There are limitations for direct use of these plasmids.
These are:
(i) Large size of vector make it difficult to manipulate
(ii) Absence of unique restriction enzymes sites and
(iii) Tumour induction.
Therefore, vectors are designed with useful characteristics. This involves-removal of tumour induction property or disarming the plasmid. This is achieved by replacing of tumour induction genes in T-DNA by selectable markers such as npt-II (kanamycine). Promoters and polyadenylation signal isolated from octopine and nopaline synthase genes were used for expression of selectable markers.
As there is no excess production of plant hormones, whole plants transformed with such disarmed Agrobacterium strains can be produced and detected by the production of opines. When a selectable marker gene (kanamycin resistant) is introduced, transformed cells can be selected by their ability to grow on media containing the selective antibiotic. Untransformed cells will not survive on this medium. Other promoters – one – CaMV35S, CaMV19S isolated from cauliflower mosaic virus have also been used. Therefore, it is concluded that T-DNA and Vir genes are two essential components of a vector.
Cointegrative Vectors:
Cointegrative vectors recombine. via DNA homology, with an intermediate cloning vector, which is used for manipulation and cloning of the gene in E. coli. Agrobacterium containing cointegrative vector and E. coli containing intermediate cloning vector are allowed to undergo conjugation, but the intermediate vector cannot replicate in Agrobacterium so it has to transfer the marker genes as well as the DNA segment to the resident Ti plasmid (cointegrative vector) through recombination in the region of DNA homology.
Example of such vector – pGV3850 from nopaline type Ti plasmid, where almost all T-DNA has been replaced by pBR322, a small E. coli cloning vector (Bolivar and Rodriguez prepared and hence name – plasmid BR, followed by experiment number). The intermediate vector (pGV1103) based on pBR322 is conjugated into pGV3850 at the region of pBR322 homology.
Binary Vector:
A significant advance that bypass the problem of Ti-plasmid size was the discovery that the T-DNA and the vir region could be separated on two different plasmids without loss of the T-DNA transfer capacity, i.e., they worked in a trans as well as a cis configuration. This discovery led to the development of binary T-DNA vectors that involve two plasmids.
The small binary T-DNA plasmid has a wide host range that can replicate both in E. coli and Agrobacterium cells. The desired foreign gene is inserted into the binary T-DNA plasmid between the left and right border sequences. A selectable plant marker gene is also inserted and (along with desired foreign gene) allow selection of transformed plant material. Several plant species have been transformed by this method.
Transformation Technique:
The critical information that made Agro-bacterium mediated gene transfer systems possible came from the elegant work by Chilton et al. (1977) who showed that in crown gall disease a region of bacterial plasmid DNA (the T- DNA) is transferred to chromosomal DNA in the plant nucleus, stably maintained there, and expressed in the absence of the bacterium.
The first transgenic Nicotiana tabacum plant was produced by Horsh and co-workers in 1984 using Agrobacterium. Agro-bacterium mediated gene transfer methods are being developed for a wide range of dicotyledonous plants and gymnosperm species. The gene tagging approach is used to demonstrate transformation.
T-DNA containing either a reporter or strong transcriptional enhancer transforms a large number of cells. Normally bacteria are incubated with plant cells (few hours to few days) during that period T-DNA transfer takes place. The cells are then washed and treated with antibiotics to remove the bacteria. The cells are then cultured in the presence of the selectable agent, and transformed shoots are regenerated and characterized. (Fig. 16.9)
Plasmids of Agro-bacterium have been used as vector for transfer of foreign DNA into a number of dicot species. However, seed legumes are still not amenable, exception is Glycine max. Monocotyledons (cereals) cannot be used as Agro-bacterium is host specific and do not infect monocots but with an exception of Asparagus (which has been transformed with this technique). There is no cambial activity and wound healing process in monocots, therefore, acetosyringone is not produced; this results in failure of vir genes to recognize by chemoreception.
(i) Pre-requisites for agro-infection:
1. Production of acetosyringone by the host plant cells.
2. Bacteria have access to actively dividing cells (DNA replication) such as meri-stems, fresh protoplasts, de-differentiated tissues.
3. Regeneration in transformed tissues should be possible; otherwise transformation will be of no use.
(ii) Explants for co-cultivation- following materials can be used:
(a) Protoplasts
(b) Cell suspension cultures
(c) Callus\thin cell layers – epidermis, tissue slice, organ section (leaf disc, section of roots, stem or flower).
(d) Wounded and inoculated whole plant.
Selectable Markers:
Selectable markers are usually required for efficient recovery of transgenic cells and plants. After gene transfer, transformed cells are few in number compared to untransformed cells. It is not possible to separate transformed and non-transformed cells by any physical method. A selectable marker gene incorporated with the desired gene helps the growth of transformed cells on a nutrient medium containing corresponding selective agent.
The availability of multiple selectable markers is useful in developing efficient transformation methods for diverse species as well as for the introduction of multiple novel traits (characters) through successful transformation and regeneration of transformed plants from such cells (Table 16.1).
Selective agents differ in their toxicity to different plant species. The different developmental states of the plant cells or tissues give different response to the selectable marker. The cells will react differently than whole plant or organ.
Therefore, it is necessary to use correct concentration of selective agent for the transformed cells of a given species to select the cells and to inhibit the growth of untransformed cells. This can be illustrated by following example.
Transgenic plants of Lycopersicon esculentum, Brassica napus and Lactuca sativa can be selected on low kanamycin concentration (15-100 mg/1), while other plants like Beta vulgaris requires high kanamycin concentration (400 mg/1) as selection agent.
Herbicides are generally more toxic to plant cells than antibiotics. This is due to their specific mode of action in plant cells as well as their efficient uptake and translocation within the plant tissues. Herbicides and other highly toxic compounds may require delayed application in order to ensure that the transformed cells have produced sufficient quantities of enzyme responsible for the protection to such compound.
Callus and cell cultures are better systems than organized explants to achieve transformations and selection of transformed cells. Explants may give rise to organs from untransformed cells which may escape selective agent. This is not possible with isolated cells and only transformed cells will be able to grow on the selective agent.
Reporter Genes:
In all transformation experiments, regeneration of non-transformed plants is also observed along with transformed plants. If these non-transformed plants can be detected at an early stage, a lot of time, labour and analyses can be saved. There are several methods to screen and identify the transformed plants, but usually these methods are laborious and time consuming, e.g. southern blotting technique to compare DNA sequences requires large amount of tissue (DNA) for comparison.
The other methods use selective agent. In both these methods, prolonged growth and subcultures are involved before selection can be made. The use of reporter gene eliminates these drawbacks and transformed plants can be recognised easily. A reporter gene is a coding unit whose product is easily assayed (such as GUS whose product can easily be detected by histo-chemical assay). This gene may be connected to any promoter of interest so that expression of the gene can be used to assay promoter function.
In fact, the reporter gene describes the transfer and expression of other promoter. Two genes that are now widely used for this purpose are those coding for β-glucoronidase (GUS) and luciferase (LUC). The coding regions of these non-plant genes have been fused to plant promoters and polyadenylation sequences such that they give high level of expression in plant cells (Table 16.2).
The anthocyanin regulatory genes can serve as a unique reporter system in maize and some other cereals. Transformed cells accumulate reddish purple anthocyanin pigments. The anthocyanin genes are extremely sensitive marker used in Arabidopsis and Nicotiana transgenic plants. Without selection agent, transformed plants are selected with the help of scoreable gene or reporter gene.
Examples of Transgenic Plants- Herbaceous Dicot – Tobacco, Petunia hybrida, tomato, potato, egg plant, Arabidopsis thaliana, lettuce, Apium graveolens (celary), sunflower, flax, rape oil seed, cauliflower, cabbage, cotton, soyabean, pea, chicory, carrot, licorice, sweet potato, kiwi, papaya, grape, rose, Chrysanthemum, etc.
Woody Dicot – Populus, Malus, Pyrus communus, Azadiraclita indica
Monocot – Asparagus, Secale cereale, Oryza sativa, Triticum aestivum, Zea mays, Avena sativa.
Gymnosperm – Picea glauca.
Method # 4. Integration and Expression:
For expression heterologous proteins in plants, the unique capacity of a soil bacterium Agrobacterium tumefaciens to introduce part of its resident plasmid into the plant genomic DNA has been widely utilized. The above bacteria causes a cancerous growth in the infected plant due to the introduction of this DNA fragment derived from the plasmid.
The plasmid is called the Ti plasmid and the introduced fragment of DNA is called T-DNA. The tumorigenic genes, i.e., those which cause the tumour are deleted from the T-DNA and then the plasmid is incapable of causing tumours but is still capable of transferring T-DNA to the plant. Any heterologous DNA can be introduced into the plant as part of the above plasmid.
To ensure high expression of the heterologous gene, strong promoters derived from plants viruses, like the cauliflower masaic virus are generally used. Several heterologous genes have been expressing plants in the recent years either to improve the field performance of crops or to impart added qualities to the edible part, like essential vitamins or even antigenic proteins, which can serve as vaccines against common disease like cholera. The low cost of growing such plants expression heterologous proteins, some of which can have high commercial values, has made expression such proteins in plants a very attractive proposition.
Several plants viruses can in fact multiply in their plant hosts to a very high level at a very short time. Some of these viruses, like the tobacco mosaic virus have been used to clone heterologous genes by replacing some of the non-essential viral genes. Such engineered viruses when allowed to infect plants, are capable of expressing the heterologous gene to very high levels in a matter of few weeks in the infected plants.
Some of the characteristic features of DNA integrations are:
1. Integration occurs at random sites in the genome.
2. Linear DNA is better integrated than circular DNA.
3. Multi-copy integration usually occurs in tandem at one site (copies attached in series).
4. Higher concentration of DNA results in high integration frequency with multiple copies of gene incorporated.
5. Use of carrier DNA (plasmid) in direct DNA transfer methods enhance multiple copy integration.