ADVERTISEMENTS:
The following points highlight the ten main physical methods of gene transfer. The methods are: 1. Biolystic or Particle Bombardment 2. Electroporation 3. Microinjection 4. Pollen Transformation 5. Liposome Mediated Transfer 6. Microlaser 7. Macro-Injection 8. Silicon Carbide Fiber (SCF) Mediated Transfer 9. Poly Ethylene Glycol (PEG) Mediated Transformation 10. Ultrasound Mediated Transfer.
Method # 1. Biolystic or Particle Bombardment:
One of the most spectacular successes in transformation of broad range of plants devoid of discrimination is the biolystic or gene gun method. This method, undoubtedly, is in driver seat among several other proposed methods.
This is the combination of biological and ballistic method. Klein (1987) has emphatically described effective and versatile particle gum method for delivering nucleic acids into intact plant cells and eventually result in transient expression of foreign gene.
ADVERTISEMENTS:
In particle bombardment mediated process, DNA coated micro-projectile is used to transform plant tissue. After being accelerated, micro projectile is propelled to pierce cell wall and membrane and enter intact plant cells. The micro projectile is small to penetrate the plant cell with limited damage and successfully introduce DNA or RNA.
Biolystic process has been used to transform larger tissue and organs such as shoot tip, leaves, callus, cotyledon, zygotic and somatic embryos. This technique was first developed in 1987, intended to transform cereals. Infact, the first genetically modified (GM) crop like maize contains Bt-toxin gene was produced by this method.
Gene Gun Design:
Particle bombardment is based on the development of gas flow system such as powder driven (PDS-1000) or helium driven (PDS-1000/Hc). Efficiency of the system depends on selection of target material, particle to be used as micro projectile and acceleration.
ADVERTISEMENTS:
Transformation efficiency depends on the amount of DNA dosage delivered into the cell, for example, low amount of nucleic acid delivery results in low transformation frequency and similarly high amount of DNA delivered into the cell leads to high copy number transformation efficiency.
In order to accomplish higher transformation rate at lower DNA concentration, the choice of chemical to coat particle have been modified, in which calcium chloride and polyamines are replaced by aminosiloxanes.
Nature and Preparation of Micro-carriers:
In the basic design of particle gun, coating of DNA onto small dense particles known as micro-projectiles is required. Several chemically inert metal particles such as gold, tungsten, palladium and platinum are employed. The size of the particles may vary between 1 and 1.6 pm in diameter.
The size of gold and tungsten particles is generally between 1 and 1.5 pm and 1.2 and 4 pm, respectively. Micro-metals are initially subjected to ethanol and sterile water washing process. Micro carrier suspension is then stored at 4°C for tungsten and – 20°C for gold particles.
Once preliminary treatment is done, micro-particles are mixed with plasmid DNA. Fixing of DNA onto the particles is carried out by either using ethanol or CaCl2 precipitation method. After precipitation, the particles are washed, resuspended and either dried or stored on ice as an aqueous suspension.
Bombardment Process:
Type I—The Original Gun Powder Charge Method:
This was originally proposed by Klein (1987) to transform epidermal cells of Allium cepa (Onion). In this method, tungsten particles of 4 pm in diameter is coated with genomic RNA of tobacco and placed on the front surface of a cylindrical nylon projectile (macro projectile) of diameter 5 mm and 8 mm in length.
ADVERTISEMENTS:
The whole projectile is prepared as a suspension in 1-2 pi of water. A gunpowder charge, detonated with a firing device is used to propel (accelerate) the nylon projectile down the apparatus. The tungsten particles move towards the steel plate, designed to stop the movement of nylon projectile.
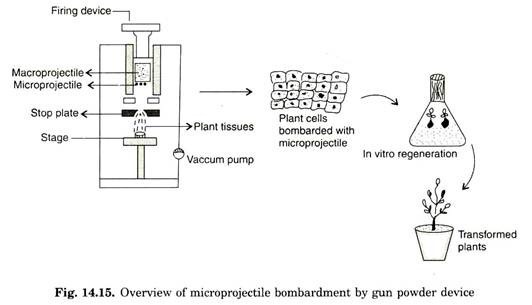
The steel plate allows the micro projectile to pass through 1 mm aperture of stop plate. Tungsten projectile leaves particle gun with an initial velocity of 430 ms-1. This high velocity can be estimated by chronograph. The target cells/tissues are placed 15-20 cm from the end of the device. Many cells are bombardment simultaneously and about 90% of the cells typically contain bombardment micro-projectiles (Fig. 14.15).
Type II—Pressured Helium Gas Bombardment Device:
ADVERTISEMENTS:
Helium blast device is a modified and upgraded version of tungsten gun powder discharge. This device was marked by BioRad as the ‘PDS-1000/He’ equipped with high-pressure helium as the source of particle propulsion.
The plasmid DNA-coated particle (micro-carrier) is placed on the front surface of the macro-carrier membrane and inserted into the apparatus. The plant tissue is placed into vaccum chamber, maintained at pressure 28 mmHg, just below the micro-carrier stopping plate. The stopping plate or macro-carrier retaining plate prevent the forward motion of the macro-projectile but designed to allow safe passage of only micro projectile.
Once partial vaccum is created at lower part of the ballistic device, pressure of the helium gas is accelerated to 1500 psi. Pressured helium gas is then released from the gas tank, and able to rupture the disc, which can resist the pressure upto 1200 psi. Following the burst of rupture disc, burst of helium gas is released. This propelled macro-carrier allows particles to move at high speed, and projectile down into a metal screen.
After macro-carrier impact with metal screen (stop plate), macro carrier is held back at stopping plate and allows micro-carrier to pass through lower chamber and finally hit plant material placed on the stage under partial vaccum. The shock wave generated after sudden release of pressured compressed gas and impact of macro projectile with stopping plate facilitate successful movement of micro-carrier and enters the plant tissue.
ADVERTISEMENTS:
Establishing vaccum in the lower chamber can reduce resistance to movement of micro-projectile by the air (Fig. 14.16). To optimize velocity of micro-projectile several parameters like distance between the stopping plate and plant material can be varied. Following bombardment plant material is transferred to suitable culture media and eventually plants are regenerated.
Merits of Biolystic Device:
i. It is efficient and easy to handle.
ADVERTISEMENTS:
ii. It can transfer genes into many cells due to multiple sites.
iii. Technique can be widely used to transform different plate material types such as culture cells, pollen, meristem, embryos, and somatic embryo. Hence, in vitro regeneration is feasible.
iv. Only cells present in the line of micro-projectile movement are killed.
v. Utility of technique can be extended to a wide group of plants including dicots and monocots.
Demerits of Biolystic Device:
i. Integration of high copy number DNA sequence into the chromosome.
ADVERTISEMENTS:
ii. Equipment costly.
iii. Cell/tissue damage due to bombardment by uncontrolled velocity of micro-projectiles.
Method # 2. Electroporation:
Electroporation is well suited for the transformation of plant cells and protoplast. Extensive work has been carried out regarding transformation of cereals using protoplast. Both linear and circular DNA can be transformed into the plant tissue. Intact plant cells of monocots have been transformed by electroporation. During electroporation, protoplast or intact plant cells are taken in electroporation chamber fitted with parallel steel electrodes.
The chamber is initially filled with buffer containing DNA of interest and high initial field strength of 1000-1500 volts with a short decay time in microseconds in applied. Pulse is applied by discharge of the capacitor across the cell. Alternatively, successful transformation is also carried out, by passing low voltage strength with larger decay of time.
Once protoplast is pulsed with low or high voltage DNA then migrated through pores into the plasma membrane induced by high voltage, eventually integrated into the genome. Most of the cereals, particularly rice and wheat have been successfully transformed by electroporation. Even other tissues such as callus and immature embryos are suggested.
Several methods have been suggested to increase transformation efficiency. Utility of osmotic buffer was well documented. Incubation of target material in high osmotic buffer before or after electroporation may increase efficiency of the technique. Addition of spermidine induces condensation of DNA, which results in enhanced efficiency of electroporation.
ADVERTISEMENTS:
Advantages:
i. Efficient transformation.
ii. Large number of transformed cells can be obtained.
iii. Production of transformants with low transgene copy number.
iv. Electroporated cells exhibit same physiological status after transformation.
v. Least number of cells deaths.
vi. Electroporation of tissue can reduce in vitro regeneration problem.
vii. Low equipment cost.
viii. Does not require experties individual.
Disadvantages:
i. Requirement of protoplast for cumbersome in vitro regeneration of plants from protoplast.
ii. Difficulties associated with regeneration of plants from protoplast.
iii. Rise of obtaining genetic variation in protoplast mediated regenerated plants.
Method # 3. Microinjection:
Transformation of higher plant cells by intranuclear microinjection has been emerged as an attractive approach in recent days. Genetic transformation of animals and insects using microinjection of embryos has been well established.
In plant system, however, protoplast is selected as favourable choice for microinjection. This technique has advanced into diverse applications in key areas like cell biology, genetics and transgenic field. Recently, microinjection is widely employed in cereal transformation.
Microinjection is a precise way of delivering genetic material into the target cells. Several workers have demonstrated the feasibility of microinjecting substances into specific cells. In order to understand intercellular transport, fluorescent dyes were microinjected. The mode of virus infection was elucidated by microinjection of viral particles into intact plant cells.
Microinjection involves direct physical approach in depositing DNA into specific target cells. Generally, microinjection requires micro-capillaries and microscopic devices to deliver DNA into cells in such a way that the injected cells survive the treatment and is able to proliferate in the cultural conditions.
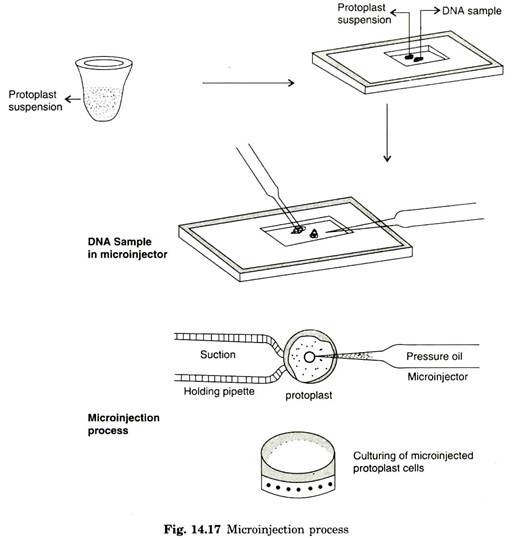
During microinjection, plant protoplast or partially synthesized cells are fixed to glass coverslips with the help of poly L. lysine. Further process requires holding pipette and micromanipulator or micro-injector. If any cell type is reluctant to attach to cover slips by binding agent, holding pipette can be an essential factor in microinjection.
These cell types are firmly retained on fixed place by blunt holding pipette. The exogenous DNA of 1 pm is taken in micro-injector and the cells or protoplasts are firmly immobilized by holding pipette by exerting suction pressure. Microinjection containing approximate dosage of DNA is then directly delivered inside the cells.
In microinjection, it is possible to microinject 200-350 protoplasts intra nuclearly and transformation frequency has been demonstrated with 20-60% success (Fig. 14.17). By means of reference marking on the coverslip, it is possible to locate microinjected cells/protoplast by recording with a video camera, which enables to work more freely from one microinjected cell to next one without interception.
Earlier microinjection studies were restricted to insect fluorescent dye and introduction of virus. Microinjection of protoplast for transformation purpose is a recent achievement. This ensures delivery of 10-3 copies of plasmid DNA into the nucleus of a particular cell type. It was however, reasonably believed that injection of DNA directly into the nucleus accelerates transformation frequency.
Advantages:
i. The amount of DNA delivered can be optimized.
ii. Precise delivery of DNA. DNA delivery is predictable even into the cell nucleus.
iii. Small cell structures like microspores, callus and proembyros can be precisely targetted.
iv. Micro-culture is accomplished.
Disadvantages:
i. Only one cell receives DNA per injection.
ii. Handling of protoplast for microinjection requires skilled persons.
iii. Sophisticated equipment.
iv. Requirement of regeneration process from microinjected cells.
Method # 4. Pollen Transformation:
Pollen approach is ideal for gene transfer into plants. It is based on the prediction that DNA can be taken up into germinating pollen and can either integrate into the sperm nuclei to reach the zygote along with pollen tube. Several experiments in established laborataries with defined marker genes produced only negative results.
Subsequent experiments however, led to successful transformation with pollen grain. Direct delivery of DNA into pollen was used to obtain transgenic Alfa-Alfa i.e., Medicago sativa. In one of the classic experiments, plasmid bearing β-glucoronidase (GUS) reporter gene was introduced into the pollen by micro-projectile bombardment.
The bombarded pollen was found to express GUS activity, when flowers of male sterile plants are pollinated with bombarded pollen containing approximate gene produced fertile seeds. Thus, transforming pollen via particle gun would be advantageous since pollen is easily available and also free cells in large number.
Most of the pollen bombarded with small tungsten particles of size 1 to 1.2 µm at a target distance of 6 cm expressed high GUS activity. Bombarding with larger size tungesten particles (1.8 µm) decreases not only the number of pollen transformation but also their germination potential.
The process of DNA delivery into the zygote via pollen tube was found to be an effective approach. Selection of ovules for gene transfer is not feasible as it poses series of challenges in the isolation of egg. One of the main apprehension of pollen transfer in that the bombarded pollen may lose its germination potential because of the mechanical aberrations occurring on the membrane and cell organelle during penetration of tungsten with high velocity (28 inch of Hg).
Tobacco pollen was transformed with GUS via particle gun method. The transgenic tobacco expressed GUS activity efficiently and it was presumed that higher vaccum, chamber presents less air resistance to micro-projectile and cause deeper peneration into the cell and their oragenelle.
Method # 5. Liposome Mediated Transfer:
ADVERTISEMENTS:
Liposomes are lipid vesicles, which are made artificially for transformation purposes. Liposomes are encircled by synthetic membrane of phospholipid. DNA-containing liposomes can be made to fuse with protoplast and have also been applied to various tissues, cell cultures and even to pollen tube with the presumption that liposomes might aid in transporting DNA via plasmodesmata directly across cell walls.
When DNA containing liposomes are induced to fuse with protoplast using polyethylene glycol, get attached to protoplast membrane. Fusion of liposomes will be resulted at the point of attachment of DNA or plasmid DNA while entering the cell. This technique has no obvious advantages over any other gene transfer methods.
DNA containing liposomes can be directly microinjected into the vacuole, releasing the content of liposome into the cytoplasm. However, micro-injected vacuole led to fusion with tonoplast. This indicates that they could be used to transform even vacuolated cells. Although this method is elegant on certain criteria, unfortunately, regeneration plants are problematic with high vacuolated cells.
Method # 6. Microlaser:
Micro laser mediated gene transfer offers advantage only in specific cases where other methods are not advantageous. This technique involves focusing micro laser beam into the light path or microscope used to burn holes into the cell wall as membrane DNA uptake is possible through penetrated cells during incubation.
Several instances have shown that DNA gets adsorbed to the cell wall material even before its entry inside the cell.
Method # 7. Macro-Injection:
Gene transfer by macro-injection may not be an ideal choice on several occasions where size of injection needle exceeds cell diameter may disrupt it. DNA integration into cell would therefore require DNA to move into wound adjacent cells.
Entry of DNA may be impossible due to closer plasmodesmata and cell wall barrier. A marker gene, however, when injected into the stem below the floral meristem shows evidence of transformation. Due to lack of reproducible and convincing evidence, this approach was found to be highly limited.
Method # 8. Silicon Carbide Fiber (SCF) Mediated Transfer:
SCF does not require any specialized equipment. In this approach, silicon carbide fibres in average of 0.4-0.6 µm in diameter and 10-90 µm long are taken along with DNA in vortex tube. Plant cells or embryos are then introduced and vortexed gently. Entry of DNA into the cell is probably due to the penetration through the cell wall and plasma membrane.
Vortexing process results in the adhering DNA to silicon carbide fibres and gained access to inside the nucleus and eventually stable integration into the nucleus genome. Thus, passing of the DNA across the cell wall has advantage over other methods.
This approach does not involve regeneration of protoplast. Presently this technique is applicable to a particular species, which produce friable nature of callus. Many cereals cannot be transformed by SCF as they produce non friable brittle nature of callus.
Method # 9. Poly Ethylene Glycol (PEG) Mediated Transformation:
Poly ethylene glycol (PEG) is inert, least toxic to cells and protoplast. This was evidenced during somatic hybrid production. Efficiency of PEG has also been extended to gene transfer process. Protoplast can uptake naked DNA by treatment with poly ethylene glycol.
Efficiency of uptake can be increased in presence of divalent cation like calcium. PEG in complex with divalent cation can disturb molecular organization of the plasma membrane of the protoplast.
Positive charges of the calcium are attracted by the negative charge of the protoplast membrane and alter its zeta potential and destabilize it. Finally DNA makes entry inside the cell and integrates into the genome. The technique not only helps in assessment of transformation, but also involve in regulating gene transfer into the plant cells. Once DNA gains entry inside the cell, it is susceptible for degradation inside cytoplasm.
Method # 10. Ultrasound Mediated Transfer:
The uptake of foreign DNA by protoplast or cells can be facilitated by imposing ultrasound. Test tube containing cells or protoplast in a buffer is made to contact by inserting tip of ultrasonic device. The ultrasonic pulse generated by ultrasonicator of 0.4 m/cm2 acoustic intensity is applied for 20-25 min.
Vigorous vibration in the medium and violent collpase of bubbles generates high hydrostatic pressure and shock wave may result in sporadic localized rupture in the membrane and it can facilitates uptake of exogenous DNA.