ADVERTISEMENTS:
In this article we will discuss about the different strategies foe gene therapy.
The term gene therapy is a broad one: it encompasses many different strategies, all of which are designed to overcome or alleviate disease by a procedure in which genes, gene segments or oligonucleotides are introduced into the cells of an affected individual.
The genetic material may be transferred directly into cells within a patient (in vivo gene therapy), or cells may be removed from the patient and the genetic material inserted into them in vitro, prior to replacing the cells in the patient (ex vivo gene therapy). Because the molecular basis of diseases can vary widely, some gene therapy strategies are particularly suited to certain types of disorder, and some to others.
ADVERTISEMENTS:
Major disease classes include:
(i) Infectious diseases (as a result of infection by a virus or bacterial pathogen);
(ii) Cancers (inappropriate continuation of cell division and cell proliferation as a result of activation of an oncogene or inactivation of a tumor suppressor gene or an apoptosis gene).
(iii) Inherited disorders (genetic deficiency of an individual gene product or genetically determined inappropriate expression of a gene);
ADVERTISEMENTS:
(iv) Immune system disorders (includes allergies, inflammations and autoimmune diseases — the inappropriate destruction of body cells by immune system cells).
A major rationale for the development of gene therapy has been the need to treat diseases for which there is no effective treatment. Gene therapy has the potential to treat all of the above classes of disorder. Depending on the basis of pathogenesis, different gene therapy strategies can be considered (Table 23.3).
Current gene therapy is exclusively somatic gene therapy, the introduction of genes into somatic cells of an affected individual. The prospect of human germ line gene therapy raises a number of ethical concerns, and is currently not sanctioned.
General gene therapy strategies (see also Fig. 23.1).
Gene Augmentation Therapy (GAT):
For diseases caused by loss of function of a gene, introducing extra copies of the normal gene may increase the amount of normal gene product to a level where the normal phenotype is restored (see Fig. 23.1). As a result GAT is targeted at clinical disorders where the pathogenesis is reversible.
It also helps to have no precise requirement for expression levels of the introduced gene and a clinical response at low expression levels. GAT has been particularly applied to autosomal recessive disorders where even modest expression levels of an introduced gene may make a substantial difference.
Dominantly inherited disorders are much less amendable to treatment; gain-of-function mutations are not treatable by this approach and, even if there is a loss-of- function mutation, high expression efficiency of the introduced gene is required: individuals with 50% of normal gene product are normally affected, and so the challenge is to increase the amount of gene product towards normal levels.
Targeted Killing of Specific Cells:
This general approach is popular in cancer gene therapies. Genes are directed to the target cells and then expressed so as to cause cell killing. Direct cell killing is possible if the inserted genes are expressed to produce a lethal toxin (suicide genes), or a gene encoding a pro drug is inserted, conferring susceptibility to killing by a subsequently administered drug. Indirect cell killing uses immunostimulatory genes to provoke or enhance an immune response against the target cell.
Targeted Mutation Correction:
ADVERTISEMENTS:
If an inherited mutation produces a dominant-negative effect, gene augmentation is unlikely to help. Instead, the resident mutation must be corrected. Because of practical difficulties, this approach has yet to be applied but, in principle, it can be done at different levels: at the gene level (e.g. by gene targeting methods based on homologous recombination); or at the RNA transcript level (e.g. by using particular types of therapeutic ribozymes — or therapeutic RNA editing).
Targeted Inhibition of Gene Expression:
If disease cells display a novel gene product or inappropriate expression of a gene (as in the case of many cancers, infectious diseases, etc.), a variety of different systems can be used specifically to block the expression of a single gene at the DNA, RNA or protein levels. Allele-specific inhibition of expression may be possible in some cases, permitting therapies for some disorders resulting from dominant negative effects.
Technology of Classical Gene Therapy:
We make a distinction between gene therapy approaches which simply rely on gene transfer and expression (which we somewhat arbitrarily describe as classical gene therapy; see Mulligan, 1993) and other approaches (targeted inhibition of gene expression in vivo and targeted gene mutation correction in vivo).
Genes can be inserted into the cells of patients by direct and indirect routes, and the inserted genes can integrate into the chromosomes or remain extra- chromosomal:
ADVERTISEMENTS:
An essential component of classical gene therapy is that cloned genes have to be introduced and expressed in the cells of a patient in order to overcome the disease. Practically, this usually involves targeting the cells of diseased tissues. However, in some cases, unaffected tissues are deliberately targeted.
For example, it is sometimes advantageous to target genes to healthy immune system cells in order to enhance immune responses to certain cancer cells and infectious agents. Additionally, genes may be targeted initially to one type of tissue while the gene products may be delivered to a remote location. For example, the myonuclei in muscle fibers have the advantage of being very long-lived.
Genetically engineered myoblasts, therefore, have the potential to ameliorate some non-muscle diseases through long-term expression of exogenous genes which encode a product secreted into the blood stream.
Two major general approaches are used in the transfer of genes for gene therapy:
ADVERTISEMENTS:
(i) ex vivo gene transfer:
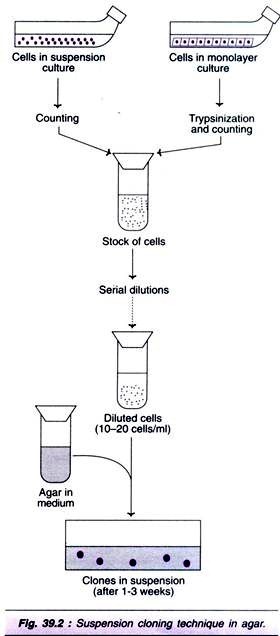
This initially involves transfer of cloned genes into cells grown in culture. Those cells which have been transformed successfully are selected, expanded by cell culture in vitro, then introduced into the patient. To avoid immune system rejection of the introduced cells, autologous cells are normally used the cells are collected initially from the patient to be treated and grown in culture before being re-introduced into the same individual (see Fig. 23.2).
Clearly, this approach is only applicable to tissues that can be removed from the body, altered genetically and returned to the patient where they will engraft and survive for a long period of time (e.g. cells of the hematopoietic system, skin cells, etc.).
(ii) in vivo gene transfer:
ADVERTISEMENTS:
Here the cloned genes are transferred directly into the tissues of the patient. This may be the only possible option in tissues where individual cells cannot be cultured in vitro in sufficient numbers (e.g. brain cells) and/or where cultured cells cannot be re-implanted efficiently in patients.
Liposomes and certain viral vectors are increasingly being employed for this purpose, in the latter case, it is often convenient to implant vector-producing cells (VPCs), cultured cells which have been infected by the recombinant retrovirus in vitro: in this case the VPCs transfer the gene to surrounding disease cells.
As there is no way of selecting and amplifying cells that have taken up and expressed the foreign gene, the success of this approach is crucially dependent on the general efficiency of gene transfer and expression.